There’s a nice potted history of the discovery of the neutron on the Nobel website. It mentions the great Ernie Rutherford who discovered the proton in 1917. He knew all about Prout’s hypothesis wherein the atomic weights of various elements were integer multiples of the atomic weight of hydrogen. However Rutherford also knew that the atomic number, the number of protons, was circa half the atomic weight. So in 1920 he suggested that this disparity was due to neutral particles called neutrons. The evidence of beta decay suggested that there were electrons within the nucleus, so Rutherford thought of the neutron as a close-coupled or paired proton-electron combination.
Problems with the nuclear-electron idea
However Werner Heisenberg said it would take 100MeV to confine an electron within a nucleus, Oskar Klein said it would tunnel out anyway, and Wolfgang Pauli said the nitrogen-14 exchange-theorem spin statistics were false. Nitrogen-14 was an integer-spin atom so it couldn’t be made up of fourteen spin ½ protons and seven spin ½ electrons. So the neutron couldn’t be made up of a proton plus an electron. The sentiment was that there couldn’t be any electrons inside the nucleus, and that electrons were created during beta decay, like photons are created when an electron drops down an orbital. That sounds fair enough when you think about the wave nature of matter. We can diffract neutrons. Diffraction occurs “when waves encounter obstacles whose size is comparable with the wavelength”. The electron Compton wavelength is 2.426 x 10⁻12 m, whilst the neutron Compton wavelength is 1.321 x 10⁻¹⁵ m. Like Martin van der Mark said in on the nature of stuff, you can’t fit a longer-wavelength particle inside a shorter-wavelength particle. That’s why there’s an issue when you try to fit a 2.3MeV quark inside a 938MeV neutron:
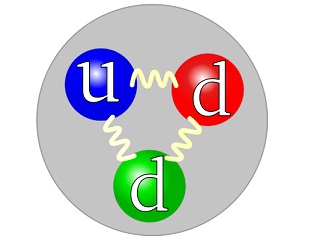 CCASA neutron image by Arpad Horvath, see Wikipedia
CCASA neutron image by Arpad Horvath, see Wikipedia
But wait a minute. the Copenhagen crew said electrons can’t fit inside neutrons, but we’re told that quarks can? Whilst attempts to model the nuclear force ended in disaster? Surely it’s time we got back to the drawing board. As Bert Schroer said: “perhaps the past, if looked upon with care and hindsight, may teach us where we possibly took a wrong turn”.
True Grit
James Chadwick had been Rutherford’s student in Manchester in 1911. In 1914 he was in Berlin using Hans Geiger’s counter to demonstrate that beta radiation had a continuous spectrum. He was only 23 at the time, and the price of his discovery was high. He spent the next four years in an internment camp where he doggedly conducted experiments with radioactive toothpaste. However the war ended, and in 1919 he followed Rutherford to the Cavendish lab in Cambridge. He became Rutherford’s protégé. He and Rutherford “often discussed neutrons, and suggested ‘silly‘ experiments to discover them”. He tried repeatedly to no avail, and the neutron was scathingly labelled the fewtron. The eventual breakthrough came more than a decade later. In 1931 Herbert Becker and Walter Bothe in Geissen fired alpha particles at beryllium to produce a very strong radiation. This radiation of beryllium was extremely penetrating: “the rays could pierce a brass plate, several centimeters thick, without any noteworthy loss of velocity. When hitting nuclei of atoms, this new radiation caused a disintegration of them, similar to an explosion”. In 1932 Irène and Frédéric Joliot-Curie in Paris demonstrated that when this radiation met paraffin it ejected high-energy protons. They thought they were dealing with 50 MeV gamma radiation, also known as quantum radiation – the word photon wasn’t so common then. Chadwick however suspected heavy neutral particles were involved, and conducted a crucial series of experiments over an intense three-week period. See his brief 1932 Nature paper possible existence of a neutron where he said this: “these results, and others I have obtained in the course of the work, are very difficult to explain on the assumption that the radiation from beryllium is a quantum radiation, if energy and momentum are to be conserved in the collisions. The difficulties disappear, however, if it be assumed that the radiation consists of particles of mass 1 and charge 0, or neutrons”.
The neutron has charge
Chadwick’s 1933 Royal Society Bakerian lecture gives further information. On page 1 he talked about neutrons moving at 3 x 109 cm/s, a tenth the speed of light. On page 12 he talked about neutrinos, though he didn’t use that name. On page 13 he said he obtained a value for the mass of the neutron of 1.0067. On page 14 he said “As I shall show later, some suggestion that either the neutron or the proton may be complex can be deduced from the collisions of neutrons with protons”. On page 18 he talked of a collision radius of 4 to 5 x 10-13 cm. What a detective, he even looked like a passable Sherlock Holmes:
 James Chadwick image from BBC News
James Chadwick image from BBC News
What really caught my eye however was this on page 2: “the collision of a neutron with an atomic nucleus, although much more frequent than with an electron, is also a rare event, for the electric field between a neutron and a nucleus is small except at distances of the order of 10-12 cm”. That’s 10-15 m. The neutron Compton wavelength is 1.319 x 10⁻¹⁵ m. Chadwick was saying that whilst the neutron is neutral, when you get up close and personal, it isn’t. The neutron has zero net charge, but it has both positive charge and negative charge, which is why it has a magnetic moment. This was determined by Otto Stern and others including Isidor Rabi in 1934. So much knowledge was already in place in 1935 when Chadwick received his Nobel prize. So so much.
Early days
Chadwick gave some interesting history in his 1935 Nobel lecture: “the first suggestion of a neutral particle with the properties of the neutron, we now know, was made by Rutherford in 1920. He thought that a proton and an electron might unite in a much more intimate way than they do in the hydrogen atom, and so form a particle of no nett charge and with a mass nearly the same as that of the hydrogen atom. Chadwick said Rutherford’s view was that such a particle was “the first step in the formation of atomic nuclei from the two elementary units in the structure of matter – the proton and the electron”. He also said that with this first step “it would be much easier to picture how heavy complex nuclei can be gradually built up from the simpler ones”. Quite, especially if you know about the alpha particle. Chadwick quotes Rutherford thus: “Under some conditions, however, it may be possible for an electron to combine much more closely with the H nucleus, forming a kind of neutral doublet. Such an atom would have very novel properties. Its external field would be practically zero, except very close to the nucleus, and in consequence it should be able to move freely through matter”. I rather think Rutherford was so far ahead of the curve that some other physicists somehow lost sight of what he was saying, and what the evidence was saying.
Heady days
Chadwick also talked about the alpha particle, saying this: “by a suitable choice of the exchange forces it is possible to obtain a saturation effect, analogous to the saturation of valency bindings between two atoms, when each neutron is bound to two protons and each proton to two neutrons”. He talked of slow neutrons, saying “the calculations of Bethe show that the chance of capture of a neutron may be inversely proportional to its velocity”. He finished up offering great promise: “These ideas thus explain the general features of the structure of atomic nuclei and it can be confidently expected that further work on these lines may reveal the elementary laws which govern the structure of matter”. Physics was making great strides, it was explaining the atom, it was explaining the periodic table. And underlying everything else was the alchemist’s dream. Transmutation. Lead into gold. Those were heady days for particle physics, for physics, for science. The Joliot-Curies missed out on the neutron, and on the positron, but they were awarded the chemistry Nobel prize in 1935. That was “in recognition of their synthesis of new radioactive elements”. Heady days indeed, for science and for the world. Did physics live up to that promise? Sir James Chadwick did. He was the grandfather of the atomic bomb. In 1941 he was on the MAUD committee. In 1944 he was at Los Alamos. He was knighted in 1945. The neutron delivered too. Sadly, it delivered death and destruction in 1945, but it also delivered peace. By 1954 we had nuclear submarines, but we also had nuclear power stations. That was a mere 22 years after Chadwick discovered the neutron.
What happened?
But now it’s 2018. It’s 64 years since the Nautilus and Calder Hall, and contemporary physics doesn’t explain the neutron and it doesn’t explain the nuclear force. What happened? Did physicists persuade themselves that it was a good idea to replace a real wave rotation with abstract mathematical symbolism? Was it because with the mistaken belief that spin is conserved in beta decay, they thought it was a good idea to introduce an abstract isospin that isn’t conserved in beta decay? Was it some kind of disregard for evidence? See the Wikipedia neutron magnetic moment article: “the existence of the neutron’s magnetic moment was puzzling”. Why? Because Pauli gave up on spin? The article goes on to say the neutron magnetic moment “defied a correct explanation until the quark model for particles was developed in the 1960s”. But the article also says “the calculation assumes that the quarks behave like pointlike Dirac particles”. Pointlike? Contemporary physics “explains” the neutron magnetic moment in terms of pointlike particles that are not explained, and have never been seen? How can the neutron be made up of point particles? It can’t be. It’s the wave nature of matter, not the point-particle nature of matter. It’s the Dirac wave equation, not the Dirac point-particle equation. Annihilate a neutron with an antineutron and you see pions, not pointlike quarks. Those pions are said to consist of quarks and antiquarks, but they don’t even last a microsecond. Circa 26 nanoseconds later you’re left with photons, electrons, and neutrinos, and contemporary physics doesn’t explain any of them. Surely we can do better than that?
Neutron properties
Yes we can do better than that, because we can explain photons, electrons, and neutrinos. And protons. And because we can use the clues from classical electromagnetism and elsewhere to come up with a description of the neutron that makes sense. For starters, the neutron magnetic moment says it has a Poynting vector, and that something is going around and around. See Hans Ohanian’s 1984 paper what is spin? He said this: “the means for filling the gap have been at hand since 1939, when Belinfante established that the spin could be regarded as due to a circulating flow of energy”. The neutron has spin, and spin is real, as evidenced by the Einstein-de Haas effect. The magnetic moment and the g-factor tells us something about it, particularly when we compare with the electron and the proton. The electron magnetic moment is −9284.764 × 10−27 J⋅T−1. The proton magnetic moment is a mere 14.10606 × 10−27 J⋅T−1. That tells us the spin is going the other way around a much smaller radius. Moreover the proton-electron mass ratio is 1836 but the electron-proton magnetic moment ratio is 658, suggesting circa three times as much major-axis rotation in the proton. The electron g-factor of -2.002 suggests a Mobius-style spin ½ rotation of 720°, twice around a twisted loop. The proton g factor of 5.585 suggests a more complex spin ½ rotation of 720°, twice around a near three-loop or trefoil path. The neutron magnetic moment is −9.662364 × 10−27 J⋅T−1, the neutron-electron mass ratio is 1838, and the electron-neutron magnetic moment ratio is 960, suggesting circa twice as much major-axis rotation in the neutron. Hence the neutron g-factor is -3.826 which is nearly twice that of the electron. That would seem to suggest the neutron has a two-loop character as opposed to the one-loop electron and the three-loop proton.
The figure-of-eight knot?
However the neutron can’t be some figure-of-8 thing because it undergoes beta decay to a proton, an electron, and an antineutrino. That magnetic moment has to be net, like the charge is net. It’s like a net rotation, like three minus one equals two. So what else can the neutron be? There is such a thing as the figure-of-eight knot. It’s the next knot in the knot table after the trefoil. It can be depicted like this:
 Public domain image by AnonMoos, see Wikipedia
Public domain image by AnonMoos, see Wikipedia
The figure-of-eight knot has a four-loop appearance, akin to a three-loop proton plus a one-loop electron. If you could pull the two larger loops outwards you’d reduce the size of the inner loops. You would have produced something that looked like it was comprised of the two major loops and not much else. This fits with what Chadwick said about one neutron binding with two protons. But he also made it clear that the neutron has charge at close quarters even though it has zero net charge. That would mean the major loops have a negative charge, whilst the interior loops have a positive charge. From what we know about the electron and positron, for this to occur the direction of the associated twist or writhe would have to change from clockwise to anticlockwise. But then surely the clockwise twist would cancel the anticlockwise twist, leaving no twist? Then we wouldn’t have a spin ½ particle. That sounds like an issue. A related issue is that the figure-of-eight knot is said to be achiral, but there is such a thing as the antineutron. The antineutron has the opposite chirality to the neutron. So the figure-of-eight knot doesn’t fit the bill.
The two-loop pion?
Is there anything else that fits the bill? Such as a pion? If a proton is comprised of three quarks and has a three-loop disposition, then surely the two-quark pion has a two-loop disposition? A figure-of-8 disposition? That sounds promising. After all Chadwick said this in his Nobel lecture: “it is assumed, with Heisenberg and Majorana, that the interaction between neutron and proton is of the exchange type – similar to that between the hydrogen atom and the hydrogen ion”. And you can read that the neutron can emit a negative pion to become a proton. John Baez and John Huerta write about this in The Algebra of Grand Unified Theories. They say that in 1932 Heisenberg proposed that the proton and the neutron were two states of the same particle. They say that in 1936 Cassen and Condon employed an analogy between “isospin” and electron spin such that the proton was an isospin-up nucleon and the neutron was an isospin-down nucleon. They say that there must be a mechanism which can convert protons into neutrons and vice versa. They say that any physical process caused by this force should be described by an intertwining operator. It’s all interesting stuff, particularly since you can “intertwine” a trefoil and an 8 to come up with something that looks promising:
 Based on a GNUFDL trefoil image by Ylebru
Based on a GNUFDL trefoil image by Ylebru
Baez and Huerta also say that “the answer originates in the work of Hideki Yukawa. In the early 1930s, he predicted the existence of a particle that mediates the strong force, much as the photon mediates the electromagnetic force”. The pion was subsequently discovered in 1947 and Yukawa was awarded the 1949 Nobel prize. Yes, the pion sounds promising, particularly since there’s such a thing as pion beta decay. That’s where a negative pion decays into a neutral pion which decays instantly into gamma photons, plus an electron and an antineutrino.
Pion exchange
However there are too many issues to claim that the neutron is some proton+pion combination. Ordinary beta decay results in a proton, an electron, and an antineutrino. Now and then there’s some internal bremsstrahlung which creates a photon, but pions are never produced. Moreover the pion is said to be spinless and described by the Klein–Gordon equation, but π+ and π− are charged particles. That doesn’t square with what we know about spin ½ standing waves and charge. The pion is also said to be composite and so not a Yukawa particle. Then there’s a problem with parity, in that pions have a parity of -1, and protons and neutrons both have a parity of +1. On top of that the neutron isn’t 140 MeV heavier than the proton, and the Yukawa interaction is an attractive force whilst the nuclear force is both attractive and repulsive. And for the cherry on top, despite what you can read on some authoritative-looking websites, pion exchange is nothing like ping pong. Pion exchange is likened to the electromagnetic interaction, but hydrogen atoms don’t twinkle and magnets don’t shine. That’s because the virtual photons that are said to mediate the electromagnetic interaction are virtual photons. They only exist in the mathematics of the model. There are no actual photons flying back and forth between the proton and the electron.
Exchange force
Besides, Chadwick said the exchange force was ”similar to that between the hydrogen atom and the hydrogen ion”. That’s where one electron is shared between two protons. The electron sticks two protons together, but it isn’t being batted back and forth. Matt Strassler said something similar in his blog post neutron stability in atomic nuclei. He said the forces between a proton and a neutron are “similar to the way the electromagnetic force can bind two hydrogen atoms into a hydrogen molecule”. Remember that a neutron can be created via electron capture, where a nucleus absorbs an inner electron. A neutrino is emitted to balance the book of spin. Then this thing that’s been created from a proton and an electron can stick two other protons together. Rather than the exchange force being something that involves messenger particles pinging back and forth, it’s more of a sharing. Two protons are both attracted to an electron. They share it. Two protons are both attracted to a neutron. They share it. Now why might that be?
Neutron charge distribution
The neutron electric dipole moment is evidence of sorts. It’s the dog that didn’t bark in the night sort, because there is no detectable neutron electric dipole moment. The neutron electric dipole moment “can only exist if the centers of the negative and positive charge distribution inside the particle do not coincide”. The magnetic moment means there’s charge in the neutron, but there’s no net charge. So there’s both positive and negative charge in a neutron, and the centers coincide. That’s interesting. As is the structure and geometry of charge distribution section of the Wikipedia neutron article. It says this: “an article published in 2007 featuring a model-independent analysis concluded that the neutron has a negatively charged exterior, a positively charged middle, and a negative core”. It refers to charge densities of the neutron and proton by Gerald Miller, which was published in Physics Review Letters in 2007. There’s an arXiv version too. It says “the results for the neutron contradict the longstanding notion, derived from both gluon-exchange and meson-cloud models [6, 7], that the non-vanishing charge density at the center of the neutron is positive”. That’s because the charge in the center is negative. It’s negative on the outside too. If you drew a plot of it, you’d see something that looked somehow familiar:
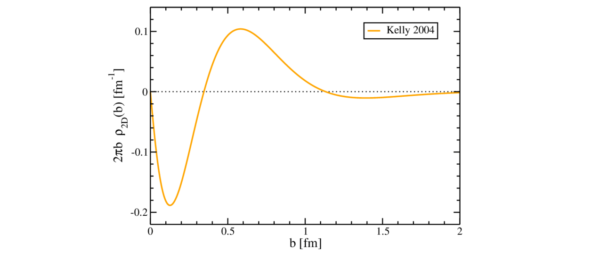 Neutron charge distribution image by Dru Renner
Neutron charge distribution image by Dru Renner
See the 2008 physics focus article journey to the center of the neutron by Don Monroe. It says this: “For decades, such experiments have implied that the neutron is a negatively-charged cloud surrounding a positive central region. But Miller’s re-analysis showed that a negative charge also exists at the core of the neutron, inside the positive region”. So the neutron has a negative exterior. That’s going to stick protons together, surely? And let’s see now. We started with a proton, which doesn’t have a negative-charge centre. Then we perform electron capture and end up with a neutron, which does. And spin is real. Oh what a tangled web we weave. You can trace it all the way back to the 1930s.
The neutron is more like the hydrogen atom than it’s like the proton
It may have been convenient for Heisenberg treat the proton and the neutron as different states of the same particle, but it’s also wrong. The proton and the neutron have a different mass and charge and magnetic moment. They are not different states of the same particle, just as the proton is not a different state of the hydrogen atom. However the difference between a neutron and a proton is one electron and one antineutrino, whilst the difference between a neutron and a hydrogen atom is one antineutrino. So the neutron is more like the hydrogen atom than it’s like the proton. But it was perceived as a different state of the proton rather than a different state of the hydrogen atom. Despite the intertwining operator, which was proposed by Casson and Condon in 1936. Despite the dihydrogen cation, which was the subject of Pauli’s thesis in 1921. Despite electron capture.
Electron capture does what it says on the tin
Electron capture is “a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shell. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino”. It was proposed by Gian-Carlo Wick in 1934 and first observed by Luis Alvarez in 1937. Alvarez was a bit of a scientific detective. So, get your thinking cap on, and ask yourself this: how does electron capture actually work? It’s one of those naïve questions for which there’s no satisfactory answer in the literature. You can find answers that say it’s because of the weak interaction. You can find answers that say the electron is converted into a neutrino. But they don’t explain what’s going on at all. In similar vein there’s no satisfactory answer for the creation of a neutron via positron emission. That occurs when the decay energy is at least 1.022 keV, which suggests that an internal pair production occurs to supply an electron which is then captured. So we’re back to the $64,000 question: how does electron capture actually work? Let’s see now. We start with an electron with a magnetic moment of −9284.764 × 10−27 J⋅T−1, and a proton with a magnetic moment of 14.10606 × 10−27 J⋅T−1. We perform electron capture, we emit a neutrino, and we’re left with a neutron with a magnetic moment of −9.662364 × 10−27 J⋅T−1. So what happened? I think there’s a vital clue the Wikipedia neutron article. In fact I think this sentence is gold dust: “This can be reconciled classically with a neutral neutron composed of a charge distribution in which the negative sub-parts of the neutron have a larger average radius of distribution, and therefore contribute more to the particle’s magnetic dipole moment, than do the positive parts that are, on average, nearer the core”. I think that makes gives enough of a clue as to what happens in electron capture:
 Based on a GNUFDL trefoil image by Ylebru and S-orbital © Encyclopaedia Britannica
Based on a GNUFDL trefoil image by Ylebru and S-orbital © Encyclopaedia Britannica
Electron capture does what it says on the tin. The electron is captured. It’s pulled into the proton, like an unfortunate worker in an industrial accident is pulled into a set of rollers. It’s drawn into the region of extreme curvature, reducing the magnetic moment and the angular momentum. Its effective diameter reduces a thousandfold, but the circulating energy can’t go round and round any faster, so the neutrino is emitted to conserve angular momentum. The electron is effectively winched into the proton. It is mangled into the proton. It “intertwines” with it, and the result is a neutron. There’s negative charge and there’s positive charge, and there’s two energy flows. One goes around and around one way. The other goes around and around the other way:
 Based on a GNUFDL trefoil image by Ylebru
Based on a GNUFDL trefoil image by Ylebru
When the neutron is in a nucleus, an adjacent proton usually keeps the electron captured. It sounds like tension on the rollers, like pulling on a rope to stop a slip-knot slipping. Chadwick was right, the neutron is complex. Rutherford was right too, because the neutron is like a close-coupled hydrogen atom. Not quite the same, but not totally different. Because what we call β– or beta-minus decay today used to be called plain old beta decay. And because in that beta decay, the beta particle is always an electron.
Beta decay is the jumping popper of particle physics
Beta decay is the jumping popper of particle physics. In a way it’s the opposite of electron capture. In electron capture the electron is captured by a proton in a proton-rich nucleus, as if they’re going to annihilate but can’t. The result is what we call a neutron. The electron usually stays captured in a proton-rich nucleus. But relocate the neutron into a neutron-rich nucleus, or kick it out of a nucleus altogether, and the surfeit of protons that caused the electron capture are no longer present. The electron is no longer constrained. It eventually works loose and resumes its original larger configuration, with the antineutrino being emitted to again conserve angular momentum. This release is perhaps something like the sudden release of a wound-up spiral mainspring. If you’ve ever toyed with clockwork you will have had a mainspring leap out of its housing and shoot across the room. I think beta decay is something similar. Kick a neutron out of a typical nucleus where it’s no longer tensioned by protons, and in about 15 minutes that neutron has decayed into a proton, an electron, and an antineutrino: n → p + e– + v̄e. The decay energy is 0.782MeV, the electron might have 0.2 MeV of kinetic energy and might be emitted at a relativistic speed of perhaps 0.7c. That electron doesn’t just fall out, it shoots out, in a direction that’s opposite to the spin alignment. The angular momentum conservation of beta decay is the time-reverse of that in electron capture, so the antineutrino truly is the antiparticle of the neutrino. So the standard model was right about something: all neutrinos are left-handed, and all antineutrinos are right-handed.
Excuse me Mr President
However the standard model says there’s a separation of positive and negative charge within the neutron because it’s made up of two down quarks with a charge of -⅓ and an up quark with a charge of +⅔. But there is no detectable electric dipole moment, and no detectable quarks either. And the standard model, as per the Wikipedia neutron article, says the main attraction between neutrons and protons is via the nuclear force, which does not involve charge. I am reminded of Independence Day. Uh, excuse me, Mr President? That’s not entirely accurate. Because we created that neutron via electron capture, and in ordinary hadrons the gluons are virtual. They only exist in the mathematics of the model. The neutron magnetic moment does not, nor does nuclear magnetic resonance, and nor do atomic nuclei.
The structure of the proton and neutron can be found in detail at the web page http://www.primons.com
I couldn’t see much on proton structure, Mario. I saw this: http://www.primons.com/category/proton-structure/ but it didn’t say much.
a) Waves are rather large in extent. How can you confine a wave of a field in a small volume?
b) Fields are larger still. What happens with the “not-wave” field between two electrons? Either it is detectable, or non-existent.
c) If the electron wave is a sort of knot or Moebius loop, what is the mechanism to produce it?
b) Why does the neutron “spring” needs 15 min (several billions of, say, electronic time units) to jump out of the “clock”?
c) That is, where from comes this inmense time interval?
Thanks
a) Waves are rather large in extent. How can you confine a wave of a field in a small volume?

 “>
“>

 “>
“>

 “>
“>
.
You don’t. A 511keV photon is an electromagnetic wave. It’s a dynamical field-variation moving at c, and it has no edge. When you wrap and trap it in a spin ½ double loop path you’re making a standing wave. The standing wave doesn’t have any edge. But now that dynamical field-variation going round and round at c looks like a standing field because the minimum and maximum field variations combine, along with all points in between, to leave you with a phase-invariant all-round standing-field. You can emulate that with paper strips. Try it:
.
.
However paper strips are not ideal. Imagine doing the above with a wave that looks like this:
.
.
You would end up with something like this:
.
.
b) Fields are larger still. What happens with the “not-wave” field between two electrons? Either it is detectable, or non-existent.
.
The electron is field. There is no billiard-ball thing in the middle. Because it’s just a 511keV field-variation going round and round such that the minimum and maximum combine all the way round to leave you with a phase-invariant all-round unit-charge standing-field. The electron is just a 511keV photon in a spin ½ double loop standing-wave configuration, and so is the positron, albeit with the opposite chirality.
.
c) If the electron wave is a sort of knot or Moebius loop, what is the mechanism to produce it?
.
A photon is a wave in space. Space waves, and where space waves, space is curved. So a photon moving through that space will take a curved path. Make a 511keV photon move through itself in a spin ½ double loop, and its path curves continuously.
.
b) Why does the neutron “spring” needs 15 min (several billions of, say, electronic time units) to jump out of the “clock”?
.
I can’t give a good answer to that. I guess it’s because it’s nearly symmetrical, but the captured electron gradually works loose if the neutron isn’t held fast in a nucleus.
.
c) That is, where from comes this immense time interval?
.
As above.
You speak in many of your articles of Standing Waves. But standing waves need a finite container, or do they?
I don’t think so Antonio. I think of a photon as something like a seismic wave in space. A seismic wave doesn’t have an outer edge. Then I think of the electron as a 511keV photon in a spin ½ standing-wave closed-path configuration. The minimum and maximum field variation combine, along with all points between, to form a standing wave that’s the same all round. There is no discernible phase change, hence it looks like a standing field. And there is no outer edge to this field.
Yes, but a seismic wave has an origin, a source. So it is not a good example. A closed EM wave cn be correct. An electron, with inertia (mass) as a photon is somewhat contradictory. But, O.K. you are happy with waves, I am happier with particles. Thanks a lot.
Particles are waves, Antonio. That’s why we can diffract electrons. See Pascual Jordan’s resolution of the conundrum of the wave-particle duality of light by Anthony Duncan and Michel Janssen. On page 47 they quote Jordan saying this:
.
“Einstein drew the conclusion that the wave theory would necessarily have to be replaced or at least supplemented by the corpuscular picture. With our findings, however, the problem has taken a completely different turn. We see that it is not necessary after all to abandon or restrict the wave theory in favour of other models; instead it just comes down to reformulating the wave theory in quantum mechanics. The fluctuation effects, which prove the presence of corpuscular light quanta in the radiation field, then arise automatically as consequences of the wave theory. The old and famous problem [of] how one can understand waves and particles in radiation in a unified manner can thus in principle be considered as solved”.
This looks interesting:
https://www.scribd.com/document/78563738/David-L-Bergman-Notions-of-a-Neutron
It dates from 2001.
As does this, which looks fairly new:
http://www.ecat-ilnuovofuoco.it/blog/unestensione-del-nuovo-modello-al-neutrone-e-al-protone/
This has been a very interesting read so far, and while I reserve any conclusions on your own theory of the knots (which I’ll admit is very appealing since it caters to a profound need for simplicity in modern physics) I was flabbergasted by your historic review of the standard model. It really is baffling how we managed to dig ourselves so deep in this hole, yet it makes sense given how human nature works.
Anyway, I’m interested in understanding how such knots actually form from the, let’s call it space field (since everything seems to be derived from the electromagnetic field). Here are my questions:
A) Are space-time and the EM field the same entity? From your language it would appear so, but then how do you explain the detection of gravitational waves? They don’t seem to have a corresponding EM wave with them.
B) You say that a space wave curves spacetime, and that is certainly true as any type of energy does. You then say that the knot appears because a 511 keV is so curved, that it ends up revolving around and around in this Mobius configuration, unless I’ve misunderstood your reply above. The problem is that linear EM waves of 511keV certainly exist, given that electron/positron pairs annihilate, so this mechanism can’t be always spontaneous. You say “Make a 511keV photon move through itself in a spin ½ double loop, and its path curves continuously.”, but provide no way of making a photon actually move through itself in a double loop.
It’s not my theory Leon. It’s a mixture of Maxwell’s “worble embracing itself”, plus TQFT, plus Thomson and Tait, plus Williamson and van der Mark and Qiu Hong Hu. I just try to give you my interpretation of what I’m getting from all the historical papers and articles, plus contemporary papers too.
.
Yes, particle physics is in a hole. The situation is grim. I’m not sure how it can get out. That’s a far bigger problem than dark matter, dark energy, how a magnet works, the nuclear force, et cetera.
.
No, space-time and the EM field are not the same entity. Space-time is an abstract thing that models space at all times, and is therefore static. Space is not. Waves run through it. We live in a world of space and motion. At the fundamental level, that’s the motion of waves in space. Take a look at William Kingdon Clifford’s space theory of matter. I think he pretty much nailed it in 1870.
.
I don’t have an issue with gravitational waves, though I am somewhat cynical about LIGO. A gravitational field is a place where space is “neither homogeneous nor isotropic”. That’s what Einstein said. That says to me that a gravitational wave is more like a longitudinal wave than a quadripole transverse wave. It’s a different sort of wave to an electromagnetic wave which is said to be a transverse wave. PS: I still don’t know the exact nature of the photon. It seems to be a twisted longitudinal wave, hence it has a circular polarization. But I’m not 100% sure about that.
.
You need to read what I said about energy. As far as I can tell, it’s the same thing as space. Hence energy curves space because it’s like injecting space into space, wherein space is like some kind of gin-clear ghostly elastic jelly. Then when space expands, the energy density reduces. There’s an energy density gradient wherever a gravitational field is. The electromagnetic field is akin to twisted space. Check out gravitomagnetism.
.
I say that when space waves, it curves space. Then a wave moving through that space follows a curved path. If that path is very very curved, the wave can end up encountering its own curved space, and then it can end up in a closed path: as a “hitch” or knot. People like de Broglie and Schrodinger talked about this, along with Charles Galton Darwin, plus Born and Infeld. Sadly nobody seems to have read their papers.
.
IMHO the 511keV photon can end up moving through itself in a double loop because a photon is a wave in space. Where space waves, space is curved. So a wave moving though that space does not follow a linear path. And there is only one wavelength where that curvature is right for the common amplitude that applies to all photons. That’s what underlies Planck’s constant, I am sure of it. The dimensionality of action can be expressed as momentum x distance, and I think it’s a real distance.
I’m not sure I follow your argument. According to this line photons are transverse waves in space, this solid-like stressable medium, while the gravitational field is basically just a stretching of the space (as in caused by gradients in space “density”). Without invoking extra dimensions in which we make space oscillate transversally, how should we define this oscillation? How can space oscillate transversally? I also still don’t see how it can loop (as a matter of fact, radiation travels linearly in flat space), unless you introduce another inhomogeneity that makes the wave “curl” around it. This is basically diffraction, where denser regions of the medium bend the light ray, but the energy content of an electron is far off to be able to produce enough curvature, so an external influence must enter the system. It is notable that pair creation ONLY happens when the photon is in the proximity of a nucleus, to be able to conserve momentum. But again, there’s not enough curvature.
In the williamson and van der mark paper (“Is the electron a photon with toroidal topology?”), the authors discuss solutions to maxwell equations where two colliding, head on photons produce stable field configurations where both E and B field are parallel and non propagating, but the results dont form any double loops because there’s no curvature involved.
Also, I don’t understand what amplitude has to do with anything. On the page “how pair production works” you derive the “amplitude of a photon” in a weird way: “The wavelength of a 511keV photon is 2.426 x 10-12 m. That’s the electron Compton wavelength. In the double-loop trivial-knot configuration you divide by 2π for the diameter, which is 3.861 x 10-13 m. That suggests the amplitude of all photons is 3.861 x 10-13 m”. Why do you pair the diameter of the loop with the amplitude? Also, wasn’t the amplitude of an EM wave measured in field strenght units? Why do you use meters? The amplitude shouldn’t be a measure of a spacial extent, but maybe you can explain this one by explaining how space can wave transversally.
I’m not sure I follow your argument. According to this line photons are transverse waves in space, this solid-like stressable medium, while the gravitational field is basically just a stretching of the space (as in caused by gradients in space “density”)
.
Yes, though I suspect the photon is twisted as per the penultimate picture on https://physicsdetective.com/the-photon/. Also, a gravitational field is a gradient in the “outward compression” of space, like the hypodermic analogy here: https://physicsdetective.com/how-gravity-works/.
.
Without invoking extra dimensions in which we make space oscillate transversally, how should we define this oscillation? How can space oscillate transversally?
.
The same way any elastic solid oscillates. For example within the Earth we have S-type seismic waves.
.
I also still don’t see how it can loop (as a matter of fact, radiation travels linearly in flat space), unless you introduce another inhomogeneity that makes the wave “curl” around it.
.
Don’t overthink this. Where a transverse wave in space is located at this moment, space is curved. Another wave moving through that space will follow a curved path.
.
This is basically diffraction, where denser regions of the medium bend the light ray
.
No, it isn’t. Gravity is a refraction (I presume diffraction was a typo). Einstein spoke of this, talking of space that was “neither homogeneous nor isotropic”, the refraction of light rays in a gravitational field, and referring to Huygens. Modern authors have spoken in similar terms, see https://iopscience.iop.org/article/10.1088/0256-307X/25/5/014/meta. Curved spacetime is inhomogeneous space, where the inhomogeneity is not uniform. It isn’t the same thing as curved space. It’s quite difficult to imagine curvature within a bulk, so instead imagine a large steel hoop suspended from a cable. Hit it with a hammer, and the sound waves in the steel follow a curved path round the hoop.
.
but the energy content of an electron is far off to be able to produce enough curvature, so an external influence must enter the system. It is notable that pair creation ONLY happens when the photon is in the proximity of a nucleus, to be able to conserve momentum. But again, there’s not enough curvature.</
.
There’s also gamma-gamma pair production. A 511keV photon provides enough curvature. Again you’re thinking that curved spacetime is the same thing as curved space.
.
In the Williamson and van der Mark paper (“Is the electron a photon with toroidal topology?”), the authors discuss solutions to maxwell equations where two colliding, head on photons produce stable field configurations where both E and B field are parallel and non propagating, but the results don’t form any double loops because there’s no curvature involved.
.
They did refer to a double loop, only they showed it looking like an 8. See the fourth image here: https://physicsdetective.com/how-pair-production-works/. However they spoke in terms of E and B fields rather than the electromagnetic field and spatial curvature. Sadly Martin is no longer with us.
.
Also, I don’t understand what amplitude has to do with anything. On the page “how pair production works” you derive the “amplitude of a photon” in a weird way: “The wavelength of a 511keV photon is 2.426 x 10-12 m. That’s the electron Compton wavelength. In the double-loop trivial-knot configuration you divide by 2π for the diameter, which is 3.861 x 10-13 m. That suggests the amplitude of all photons is 3.861 x 10-13 m”. Why do you pair the diameter of the loop with the amplitude? Also, wasn’t the amplitude of an EM wave measured in field strength units? Why do you use meters? The amplitude shouldn’t be a measure of a spatial extent, but maybe you can explain this one by explaining how space can wave transversally.
.
This goes back to William Kingdon Clifford’s space theory of matter, and to Maxwell’s transversion undulations. Again don’t overthink it. Instead look for modern authors saying the same thing, like Percy Hammond. Think electromagnetic geometry. Einstein said a field is a state of space. What’s the state of space where a gravitational field is? Inhomogeneous, not curved. What’s the state of space where an electromagnetic field is? Curved. Then ask yourself this: if space is waving, how much is it waving? Also ask yourself what underlies the h in E=hf, and why is the dimensionality of action momentum x distance? As far as I can tell it’s something like plucking a Spanish guitar. Your left hand moves up and down the frets so the wavelength varies. But your pluck doesn’t change, so the amplitude doesn’t change either.
Hello, J.D., Ph.D,
My favorite physics-detective articles are those pertaining to the neutron and the nuclear force. With these postings, you have converted a long-standing who-done-it into a why-of-course-it’s-obvious. This is soothing to my mind.
Question A: As you have elucidated, each of the stable elementary particles, namely, electron, proton, and neutron, constitutes at least one photon traveling along a closed loop twisted path. The neutron and the hydrogen atom each include two such solitons. The 2-neutron helium atom must comprise 8 trapped solitons. Can you say anything about the state of space where elementary particles are tethered to one another by the electromagnetic force? For example, do the standing waveforms of the proton and electron combine to produce secondary or derivative standing waveforms within the hydrogen atom and the neutron, in the space between (and outside) the positively charged “particle” and the negatively charged one? What would be the geometric difference in the spatial vibratory state of the neutron versus the hydrogen atom?
Questions B: Would you say that an electron and a nucleus together naturally generate a basic or lowest-energy standing wave pattern and that different orbitals or valence states (old chemistry word) constitute different standing wave patterns, in part characterized by different frequencies and higher energies? The absorption of a photon of appropriately high energy up-shifts an electron state to a higher energy standing wave pattern. But wouldn’t energy absorption result in a higher frequency, and therefore a smaller wavelength, and a smaller spatial extent? It seems that higher-order orbitals (an old term) should mean more compact spatial regions? Like the proton occupies a smaller spatial region than an electron.
Question C: In a neutron star, how can the neutrons be compressed together so densely – wouldn’t repulsion owing to the electron shell component of the neutrons would tend to push the neutrons apart? Would you say that in the “neutronium” state , the electrons of the neutrons are bound to the protons of adjacent neutrons, in a kind of nuclear crystal?
Thanks Neil. To try to answer your questions:
.
A: yes, I think the helium atom comprises 8 trapped solitions, two protons, two electrons, and two neutrons, each of which appears to be a close-coupled proton-electron combination. I think of a particle such as an electron or a proton as topological knot of twisted space, featuring a wave or “pressure pulse” trapped in a closed path by its own spatial distortion, and the result is a kind of a vortex. Then counter-rotating vortices attract and co-rotating vortices repel. They go around one another too. I also think that in the simplest atom, the hydrogen atom, the electron sits around the proton, and does not orbit it like a planet orbits the Sun. The various orbitals denote the electron wave path. I don’t think the standing waveforms of the proton and electron combine in the hydrogen atom. I think they always stay separate, even though they;re close-coupled. There’s a combination going on of you throw a photon at the hydrogen atom. A portion of its waveform combines with the electron waveform such that the electron’s closed path changes. The same sort of thing happens in Compton scattering, but in that situation the electron simply moves, because the closed path is no longer symmetrical. IMHO there is no space between the positively charged particle and the negatively charged particle. It’s all just waves and twisted space. I’m not sure of the geometric difference in the spatial state of the neutron versus the hydrogen atom. But I think the electron is threaded through and around the trefoil loops of the proton. It’s not just wrapped and trapped by itself, but wrapped and trapped by the proton too. Like it’s stuck in the works, and so the curvature of its path is tighter. But give it 15 minutes with no bracing by surrounding nucleons, and it will work its way loose.
.
B: I would say that an electron and a nucleus together generate a low-energy standing wave pattern. The electron in a hydrogen atom has less mass-energy than a free electron, which I think is because the curved space of the proton helps to close the electron loop. Yes, I think different orbitals or valence states constitute different standing wave patterns, and that these feature different frequencies and energies. Yes, the absorption of a photon shifts an electron into a higher-energy standing wave pattern, and a smaller wavelength. But as a result the electron moves, only it can’t escape the nucleus, and the result is a wave in say a cloverleaf disposition. The wavelength is not the length around the orbital. The electron doesn’t really have a spatial extent because the electron’s field is part of what it is. Ditto for a proton. Think in terms of hurricanes with different sized eyes.
.
C: In a neutron star the neutrons can’t be compressed totally because of the neutron degeneracy pressure. I think of this as a “two vortices cannot overlap” Pauli exclusion repulsion working at smaller distances. Hence we no longer call it electron degeneracy pressure, and it’s stronger. I don’t know if in a neutronium state the electrons are bound to the protons of adjacent neutrons. The nuclear force binds protons and neutrons in a typical atomic nucleus, but we have no atomic examples of it binding protons and protons, or neutrons and neutrons. So I don’t know if it could bind neutrons and neutrons a neutron star. Maybe it happens if you increase the pressure.
Hello, John,
I provide some continuation of our recent exchanges regarding particle ontology. I copy your comments below. My responses to your comments are within brackets [ ]. I have edited out some of our prior discussion to eliminate material inessential to the progression..
You wrote: I think of a particle such as an electron or a proton as topological knot of twisted space, featuring a wave or “pressure pulse” trapped in a closed path by its own spatial distortion, and the result is a kind of a vortex.
[I do not recall that you have previously described an elementary particle this succinctly, in one sentence. Nice.]
You wrote: Then counter-rotating vortices attract and co-rotating vortices repel. They go around one another too.
[Does this sentence describe the close-coupled electron and proton in a hydrogen atom? If so, I do not see how this is consistent with your next sentence.]
Your next sentence: I also think that in the simplest atom, the hydrogen atom, the electron sits around the proton, and does not orbit it like a planet orbits the Sun.
[What does it mean to “sit around the proton”? Are you saying that the electron does not move around the proton, and that its constituent soliton retains its twisted double loop path? It seems to me that the electron can’t just sit in a fixed or static position relative to the proton. This is a dynamic system. The two solitons’ pressure waves must interact mechanically to keep the electron and the proton tied together.]
Your write: The various orbitals denote the electron wave path.
[So, the electron does move relative to the proton; it does not sit? Are you saying that the topological knot of the electron moves as a unity along the paths? Or is the electron smeared out as spatial vibrations along the paths?]
Your wrote: I don’t think the standing waveforms of the proton and electron combine in the hydrogen atom. If the standing waveforms remain separate and unaffected by one another I think they always stay separate, even though they;re close-coupled.
[How are they close-coupled if not through a composite topological configuration of space? They must act on one another. It seems to me that the standing waveforms of the proton and electron must intercalate or interdigitate in the region between the proton and electron, and maybe even outwardly of the electron. The vortices propel each other round, like a siphoning Venturi effect. This must involve some sort of interactive conformation of space: the localized topological knots of twisted space must cooperate to generate the electrical force, a vorticial force. It seems to me further that the two “particles” retain their independent localized topological knots of twisted space, for the most part, but still there must be a slight variation in the path of the electron soliton, causing that soliton to change its path about the proton, pursuant to the electrical charge force.] \
You wrote: There’s a combination going on of you throw a photon at the hydrogen atom. A portion of its waveform combines with the electron waveform such that the electron’s closed path changes. But the electron coupled to the proton is not traveling in a straight line, it’s path changes.
[Yes, the electron does not sit; rather it moves. This path change must occur owing to the proton affecting the path of the electron’s photonic soliton, which is interpreted as the attractive electrostatic/electromagnetic force on the electron, keeping it bound to the proton.]
You wrote: I’m not sure of the geometric difference in the spatial state of the neutron versus the hydrogen atom. But I think the electron is threaded through and around the trefoil loops of the proton.
[Now that’s a vision! A problem for a computer simulation of topological space. It seems to me that computer simulation and video graphics should be able to show this and other standing wave conformations of elementary particles.]
Your wrote: It’s not just wrapped and trapped by itself, but wrapped and trapped by the proton too. [Sounds good.] Like it’s stuck in the works, and so the curvature of its path is tighter. But give it 15 minutes with no bracing by surrounding nucleons, and it will work its way loose.
[And the neutron disintegrates.]
.
Your wrote: The electron in a hydrogen atom has less mass-energy than a free electron, which I think is because the curved space of the proton helps to close the electron loop.
[Can you elaborate on what you mean here about closing the electron loop? I would say that the differential in electron mass energy goes into the reconfigured space of the electron and proton close-coupled unity. This energy difference would be small, I assume, relative to the photonic soliton energy of the electron.]
Your wrote: Yes, the absorption of a photon shifts an electron into a higher-energy standing wave pattern, and a smaller wavelength. But as a result the electron moves, only it can’t escape the nucleus, and the result is a wave in say a cloverleaf disposition.
[Excellent! The configuration of higher valence states. You’ve shown this, I think, in some of the drawings you’ve posted. Thus, you have explained a long-standing staple of chemistry in the accommodation of shorter-wavelength higher-energy electron states.]
You wrote: The wavelength is not the length around the orbital.
[Sorry, I don’t get this.]
You wrote: Think in terms of hurricanes with different sized eyes.
[Speaking of that, your description of the electron includes the expansion, as it were, of the formative soliton’s toroid into a fattened, spherical geometry. (Hurricane’s eye is squeezed shut.) How does this work for the proton, with its trefoil configuration? How does a trefoil proton soliton path generate an approximately spherical configuration?]
.
You wrote” In a neutron star the neutrons can’t be compressed totally because of the neutron degeneracy pressure.
[Your answers to my questions help me differentiate between the real and the imaginary in the explanations of contemporary particle physics. “Degeneracy pressure” means something real, then. Thanks. As a counter-example, boson exchange is fanciful.]
You wrote: I think of this as a “two vortices cannot overlap” Pauli exclusion repulsion working at smaller distances.
[Yes, so, the Pauli Exclusion Principle as to fermions is real. Is it that rotational motions cannot combine?]
NS
Neil: Thanks re the “succinctly” compliment. Yes, I think the vortices sentence applies to the electron and the proton in the hydrogen atom. But subatomic particles aren’t exactly like whirlpools. The analogies I use to try to describe things aren’t perfect.
.
What does it mean to “sit around the proton”? Are you saying that the electron does not move around the proton, and that its constituent soliton retains its twisted double loop path?
.
I’m saying it’s more like Saturn’s rings than a planet orbiting the Sun. The electron is still a photon in a closed double-loop path, but it’s not exactly the same path, because the electron mass is reduced by 13.6eV. I think of the electron motion as something like that of a hula hoop. Sorry, it’s another crap analogy. But not as crap as the planet orbiting the Sun.
.
It seems to me that the electron can’t just sit in a fixed or static position relative to the proton. This is a dynamic system. The two solitons’ pressure waves must interact mechanically to keep the electron and the proton tied together.
.
Agreed.
.
So, the electron does move relative to the proton; it does not sit? Are you saying that the topological knot of the electron moves as a unity along the paths? Or is the electron smeared out as spatial vibrations along the paths?
.
Neither. The electron is a photon in a closed double-loop path, usually with a spherical symmetry. The various orbitals are where the photon path is still a closed double-loop path, distorted and stretched out into say a cloverleaf disposition.
.
How are they close-coupled if not through a composite topological configuration of space? They must act on one another. It seems to me that the standing waveforms of the proton and electron must intercalate or interdigitate in the region between the proton and electron, and maybe even outwardly of the electron. The vortices propel each other round, like a siphoning Venturi effect. This must involve some sort of interactive conformation of space: the localized topological knots of twisted space must cooperate to generate the electrical force, a vorticial force. It seems to me further that the two “particles” retain their independent localized topological knots of twisted space, for the most part, but still there must be a slight variation in the path of the electron soliton, causing that soliton to change its path about the proton, pursuant to the electrical charge force.
.
I agree with that. The point I was trying to get across is that they retain their individuality. There’s still two knots, not one more complex knot.
.
Yes, the electron does not sit; rather it moves. This path change must occur owing to the proton affecting the path of the electron’s photonic soliton, which is interpreted as the attractive electrostatic/electromagnetic force on the electron, keeping it bound to the proton.
.
I agree with that too!
.
Now that’s a vision! A problem for a computer simulation of topological space. It seems to me that computer simulation and video graphics should be able to show this and other standing wave conformations of elementary particles.
.
Yes, I’ve suggested this in various places in the articles. It would be great to be able to model all this stuff and “see” it.
.
Can you elaborate on what you mean here about closing the electron loop? I would say that the differential in electron mass energy goes into the reconfigured space of the electron and proton close-coupled unity. This energy difference would be small, I assume, relative to the photonic soliton energy of the electron.
.
I thought I’d said something about this somewhere, but can’t find it. I’ve got to go shortly, but for now, it’s something like this: the electron is a 511keV photon in a closed path. If you were to try to make an electron with a photon that was 13.6 eV less 511keV, you would fail, because the curvature isn’t enough to maintain the closed path. But if you could hook it round a proton, you now have enough curvature to maintain the closed path. But now the electron is moving like a hula-hoop around the proton.
.
You wrote: The wavelength is not the length around the orbital. Sorry, I don’t get this.
.
It’s tricky to explain. I’ll have to come back to this another time.
.
Speaking of that, your description of the electron includes the expansion, as it were, of the formative soliton’s toroid into a fattened, spherical geometry. (Hurricane’s eye is squeezed shut.) How does this work for the proton, with its trefoil configuration? How does a trefoil proton soliton path generate an approximately spherical configuration?
.
The same way. Try drawing a trefoil with a thin thread. Then with a fatter thread. Then a much fatter thread.
.
Your answers to my questions help me differentiate between the real and the imaginary in the explanations of contemporary particle physics. “Degeneracy pressure” means something real, then. Thanks. As a counter-example, boson exchange is fanciful.
.
Yes, boson exchange is cargo-cult physics. Read Cathryn Carson’s two papers.
.
Yes, so, the Pauli Exclusion Principle as to fermions is real. Is it that rotational motions cannot combine?
.
They can combine after a fashion, but they cannot overlap. Sorry, I have to go.
This guy did a fair job of explaining wave nature of electron id recon.
.
MLA style: The Nobel Prize in Physics 1929. NobelPrize.org. Nobel Prize Outreach AB 2024. Sun. 1 Sep 2024.
.
https://www.nobelprize.org/prizes/physics/1929/summary/
Agreed Steve. Sadly it seems to have been forgotten, and nowadays physicists will tell you the electron is a point particle.
Re wavelength around electron, this guy had some decent thoughts…:
.
https://www.nobelprize.org/prizes/physics/1929/summary/
.
Did you delete this comment Steve? It was in the bin.
On page 5 he mentions the refractive index of the vacuum.
.
https://www.nobelprize.org/uploads/2016/04/broglie-lecture.pdf
.
Yes. It’s a pity he didn’t think about that more, or read what Einstein said about gravity being a refraction. Then he would have realised that the field of force is only there because of the refraction. Well, when it comes to gravitational force that is. Electromagnetic forces are different.
https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/
.
Talk about a quantum leap!
Hi John,
I think you are throwing away the figure 8 knot a bit too rashly. The particle antiparticle dilemma isn’t really a disqualifier, particular given the paucity of anti-neutron technologies/experiments. It’s rather possible that Bruce Cork was misinterpreting his data and nobody has really bothered to follow up or call him out as the ‘every particle must have an antiparticle’ mantra is tauted even now, when zoo particles are allowed to be their own antiparticle, aka mirror image.
This is particularly true given what a figure 8- tetrahedral neutron gives you in terms of explaining the Segre Chart data. The proof is in the pudding.
There are a few other folks who have picked up on the Kelvin hypothesis, it’s only a matter of time until the collective grasps the elephant in better detail….
Maybe, Etherdias. But the more I think about it, the more I think the neutron is a close-coupled electron-proton combination. Like Rutherford said but with a neutrino twist. Read the next article, and see the bit about the neutron charge profile matching the nuclear force plot. The neutron is usually kept strapped down tight in a nucleus, but if the neutron gets out, the electron shakes itself loose in maybe 15 minutes, and pop, beta decay happens. So the neutron looks like two intersecting knots to me, not one. Noted re Bruce Cork etc, and the collective.
https://docs.google.com/document/d/15MGz_d8p424xmTwKmKGu99zyl0cSvTvY/edit?usp=drivesdk&ouid=111833488969055296879&rtpof=true&sd=true
I hope this transfers, if not please reformat /delete per John’s discretion.
Better yet, I hope it might make mathematical sense. YAY ? or NAY ?
I had to request access. Bah.
Will resend via email. It was completely done on ChatGPT brand of A.I..
It’s nothing but a condensed version of your own The Physics Detective articles, with a few added fundamental paradigms.
The text reads as highly plausibe, but the maths are probly a stretch in imagination at best; total bollocks at worse !
John, again your descriptions and combinations of work is remarkable. Again this makes alot of sense, particularly regarding electron capture and beta decay as well as explaining stabilizing effect of neutrons to protons in a nucleus. I mean in electron capture a proton is converting into a neutron, why do we have to try to bring magical thinking or unexplained phenomenon, or incomplete fantastical models? This is a simpler and rational explanation. I would suggest that physicists should go about probing and testing this model prospectively.
One area where I am still grappling and don’t fully agree with is your concept of these trapped/looping photonic waves having no boundary? I think the charge field can emanate out, but I think of the construct itself (electron knot, proton knot, neutron knot) as being constrained and confined in size, like a spinning gyroscope. Now regarding electron orbitals in classical chemistry, its not that the gyroscope wave pattern changes to a different orientation (because that would be destabilizing), but instead the entire gyroscope starts moving as one piece in space in a complex orbit around the nucleus. So it is a constrained wave moving in a wave pattern. Contact me if you want to discuss further, I have some interesting ideas regarding how these knots could be formed.
Andy: many thanks. Re the waves having no boundary, take a look at the photon and check out the blue ocean-wave gif. The wave motion extends deep into the ocean. A wave in space would be something like that both above and below, because there’s no horizontal surface. If you then wrap it and trap the wave in a closed path and then call it an electron, it bears some resemblance to a hurricane. There’s a path length which is related to the wavelength. This is like the circumference of the eye of the hurricane. But there’s no outer circumference, and no outer limit to the electron electromagnetic field. Which is really just a wave in a closed path, which looks like a standing wave. Hence standing wave standing field. Only it isn’t actually motionless. The spin is real, hence Larmor precession of that spin is why the electron goes round in circles in a uniform magnetic field. As I said, I don’t know much about orbitals. But I always want to discuss things further. Do I have your contact details? Apologies if I’ve missed them. Life has been hectic of late.