The nuclear force holds atomic nuclei together. When protons and neutrons are a femtometre apart, the nuclear force between them is powerfully attractive. If you could turn this powerfully attractive force off, an atomic nucleus would explode into a spray of protons and neutrons. That’s because there’s an electromagnetic force between the protons, and it’s powerfully repulsive. In stable nuclei, the forces are in balance. But as Rod Nave says on his most excellent hyperphysics website, when the balance is broken the resultant radioactivity yields particles of enormous energy:
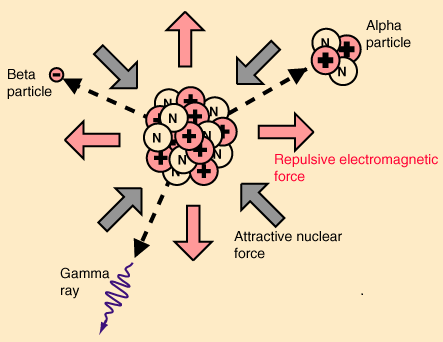 Image from Rod Nave’s hyperphysics
Image from Rod Nave’s hyperphysics
At larger distances the repulsive electromagnetic force between two protons is the same as the repulsive force between two electrons. The expression for this force can be written as F = k q²/r², where k is Coulomb’s constant, q is unit charge, and r is the separation. However the electron’s Compton wavelength is 2.426 x 10-12 m, whilst the proton’s is 1.321 x 10-15 m. Because of this, two protons can be more than a thousand times closer together than two electrons can ever be. That means the electromagnetic force between them can be a million times stronger. As for how strong this is compared to the proton-neutron attractive force, note that elements with an atomic weight greater than lead are said to be unstable, and that the lead-204 nucleus is comprised of 82 protons and 122 neutrons. That’s food for thought.
The nuclear force is not a settled matter
Especially since the nuclear force is often said to be due to a pion exchange as proposed by Hideki Yukawa in 1935. However the Yukawa interaction is inadequate to describe the nuclear force, even after a variety of attempted fixes to extend the original charged pions to mesons in general. The nuclear force is also said to be a residual effect of the strong interaction that binds quarks together, such that the meson-exchange concept is no longer perceived as fundamental. However there is no QCD model for the nuclear force. Hence the nuclear force is “not a settled matter”. So here we are a hundred years after Rutherford discovered the proton, and the nuclear force still appears in the list of unsolved problems in physics. It’s something of a disaster. As for the reason, I think it’s safe to say it’s because contemporary particle physicists do not understand the electron, or the proton, or the neutrino, or the neutron.
Rutherford’s composite neutron
Back in 1920 Rutherford knew that the atomic number of various elements was circa half the atomic weight. He suggested the disparity was due to neutral particles called neutrons, and he thought of the neutron as a close-coupled proton-electron combination. Other physicists said it couldn’t be, because it would take 100MeV to confine an electron within a nucleus, or because it would tunnel out, or because the spin statistics were incorrect. However in 1933 James Chadwick said “the electric field between a neutron and a nucleus is small except at distances of the order of 10-12 cm”. Chadwick didn’t say pion field or strong-force field, he said electric field. He was talking electromagnetism. Talking of which, a year later in 1934 the neutron magnetic moment was determined by Otto Stern, Isidor Rabi and others. It isn’t zero so it doesn’t conform to the Dirac particle expression μN=eħ/2mp. This indicates that the neutral neutron is not elementary and is instead composite like Rutherford said. On top of that the hard scientific fact of beta decay makes it plain that the neutron is more like the hydrogen atom than the proton. The difference between a neutron and a proton is one electron and one antineutrino. The difference between a neutron and a hydrogen atom is one antineutrino. But somehow this was overlooked, as was the neutron magnetic moment itself.
That composite neutron has spin
The neutron magnetic moment says there’s a Poynting vector wherein energy is circulating around and around. That’s as per Hans Ohanian’s what is spin: “the means for filling the gap have been at hand since 1939, when Belinfante established that the spin could be regarded as due to a circulating flow of energy”. Unfortunately Belinfante’s paper was overlooked too. Hence the Wikipedia neutron magnetic moment article says “the existence of the neutron’s magnetic moment was puzzling and defied a correct explanation until the quark model for particles was developed in the 1960s”. The correct explanation is that the magnetic moment is there because the neutron has spin, and because spin is a real rotation as evidenced by the Einstein-de Haas effect. But in the thirties physicists thought it was a good idea to abandon spin in favour of abstraction. They still think it’s a good idea to say the strong interaction between all nucleons is the same, even though there are no diprotons or dineutrons. Some might point to reports of a tetraneutron, but when it lasts a billionth of a trillionth of a second it just doesn’t count. An “inferred” existence is just not the same as a stable particle, regardless of some five sigma hype or any other damn statistics.
The charge independence hypothesis is not justified
The moot point is that electrons stick protons together to make hydrogen molecules, and neutrons stick protons together to make nuclei. In the former situation you can’t stick two protons together without an electron, in the latter you can’t stick two protons together without a neutron. Another moot point is that you performed an electron capture to convert a proton into a neutron in the first place. You can then use that neutron to make a deuteron, because a proton will stick to a neutron. But a proton won’t stick to a proton. You can’t make a nucleus out of two protons, or three or four or more, even though the strong force is said to be stronger than the electromagnetic force. How the theory of scattering of protons by protons and the charge independence hypothesis trumped that hard scientific fact I don’t know. But I do know that in what holds nuclei together, Matt Strassler said neutrons are needed to make protons stick together, and protons are needed to make neutrons stick together. So why might that be? What is it about the neutron that sticks protons together?
Neutron charge distribution
The neutron has zero net charge like the hydrogen atom, but there’s charge in there all right. As per the Wikipedia neutron article, the magnetic moment “can be reconciled classically with a neutral neutron composed of a charge distribution in which the negative sub-parts of the neutron have a larger average radius of distribution”. The neutron has more negative charge on the outside, and more positive charge on the inside. Opposite charges attract and like charges repel because charged particles are spinors. It’s like the way counter-rotating vortices attract and co-rotating vortices repel. If you were a proton and you were close to that neutron, you’d feel an attraction towards it. If however you got too close to the more-positive core, you’d feel a repulsion. Alternatively if you were to move away, the neutron’s positive and negative charges would tend to cancel and you wouldn’t feel anything. The force would therefore be short range. As Eliyahu Comay said in an invitation to solve a mystery, the nuclear force between nucleons shares some marked similarities with the van der Waals force between molecules. It’s repulsive at short range, it isn’t felt by particles when they’re far apart, and the force potential looks like a ski jump. What’s not to like?
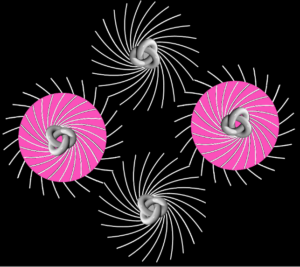
A tensor force
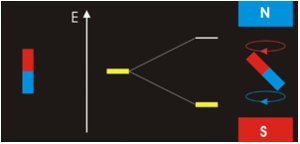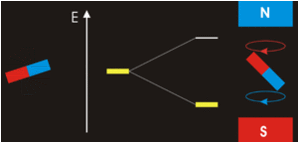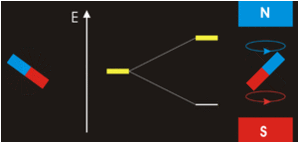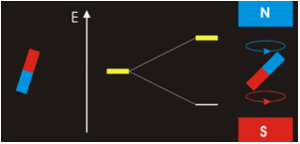
Especially since we’re dealing with spinors so there’s a rotational magnetic force too. A tensor force. The electromagnetic field has a tensor character. In Ruprecht Machleidt’s 2013 CNS summer school lecture you can read that two bar magnets provide an example of a tensor force:
 Image by Ruprecht Machleidt
Image by Ruprecht Machleidt
It all seems perfectly straightforward. In the spin-1 deuteron the proton-neutron spins are parallel. It’s as if each spin is around an axis and each axis points in the same direction, like the North poles of the top-to-tail bar magnets. This spin-1 state is the lowest-energy state. It’s unlike the proton-electron spins in hydrogen. That’s where the spin-0 antiparallel state is the lowest energy, because “the electron is not spatially displaced from the proton, but encompasses it”. It’s akin to a smaller magnet being situated in the gap between the poles of a much larger magnet. Then as per the Wikipedia nuclear magnetic resonance article, there’s a high-energy state and a low-energy state, depending on the orientation of the smaller magnet within the field of the larger magnet. The low energy state is when the North pole of the smaller magnet is below its South pole and the North pole of the larger magnet is above its South pole, like so:
 CCASA image by Darekk2 , see Wikipedia
CCASA image by Darekk2 , see Wikipedia
They don’t point in the same direction, so their spins are antiparallel. But with the addition of some judicious energy, the smaller magnet can be inverted. It can then undergo a spin-flip back to its original orientation, releasing the energy. See the Wikipedia electron paramagnetic resonance article: “the basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but it is electron spins that are excited instead of the spins of atomic nuclei”. The bottom line is that nuclear magnetic resonance occurs because the neutron shares some similarity with an electron, and with a magnet. That’s hard scientific evidence for the electromagnetic nature of the neutron. There’s more hard scientific evidence in decay and annihilation.
Rendering down
A neutron decays into a proton, an electron, and an antineutrino, which departs at the speed of light. Then you can perform proton-antiproton annihilation and electron-positron annihilation to produce gamma photons which again depart at the speed of light. Yes, you can also produce pions in proton-antiproton annihilation, but “they will decay in a series of reactions that ultimately produce only gamma rays, electrons, positrons, and neutrinos”. If you then perform electron-positron annihilation, your final end products are gamma rays and neutrinos which depart at the speed of light. So what happened to the fundamental quarks and antiquarks? They allegedly went to make up the pions. That sounds reasonable enough. But what do we actually see? Any number of pions. Perhaps eight. That’s more than the supposed number of quarks in the proton plus antiproton. See the LBL account of the antiproton experiments of Segrè and Chamberlain et al on the Bevatron in 1955:
 Image from the particle adventure
Image from the particle adventure
Thus using the logic of the thirties that said the electron is created in beta decay, the proton isn’t a three-quark combination, and the neutron isn’t a proton plus a pion. It can’t be, because the pions that are said to mediate the nuclear force are virtual, as in not real, as in not there. It’s the same for the gluons that are said to mediate the strong force. The gluons in ordinary hadrons are virtual, as in not real, as in not there. How can they be fundamental if they’re not there? And what happened to the supposedly fundamental quarks? We allegedly had six, then any number, then none. Or did we start with an infinite number of quarks? Were any of these things ever there? As discrete particles, no. But something else was. You can diffract protons and neutrons, because of the wave nature of matter. Because of the standing wave nature of matter. Their spin is real, and charged particles attract and repel because they’re spinors. Because of the screw nature of electromagnetism. Then when you annihilate two opposite spinors they aren’t standing waves any more. They’re waves of a different type. Waves which depart at the speed of light. Electromagnetic waves. Because what was always there, was electromagnetism.
Electromagnetic spinors
Electrons move the way that they do because they’re dynamical spin ½ “spinors”. It takes two to tango, and the result is linear electric force and rotational magnetic force. Protons and neutrons are dynamical spin ½ spinors too. Throw a proton through a solenoid, and its path is helical. Throw a neutron through a Stern-Gerlach magnet and its path curves. Because spinors are what they are because spin is real. There are no gluons flying around, and there are pions flying around, because those gluons and pions are virtual. But electron capture is not virtual, and nor is the force between the proton and the neutron. It’s real, as is the neutron’s magnetic moment, and the neutron’s charge distribution. The neutron has more negative charge on the outside, and more positive charge on the inside:
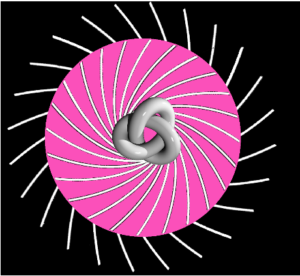 Based on a GNUFDL image by Ylebru
Based on a GNUFDL image by Ylebru
That’s surely going to mean that protons and neutrons will interact, and will be subject to linear and rotational electromagnetic forces. And with both particles having Compton wavelengths of circa one femtometre, the forces will be large compared to the forces between electrons and positrons. But they will be similar. Which is why in his Nobel prize lecture Yukawa said the force between a neutron and a proton was like the bond between a hydrogen atom and a proton. Yes it is, because it’s electromagnetic. That’s why when you plot it, it resembles the hydrogen molecule plot. That’s surely why the neutron is something that can bind two protons but no more, like the electron in the dihydrogen cation. That’s surely why the nuclear force has a tensor character, like the electromagnetic field has a tensor character. That’s surely why we have such a thing as the nuclear spin-orbit interaction. The idea sounds reasonable. So let’s take it for a spin and see if it flies for a few simple nuclides.
The deuteron
The first nuclide is of course protium, a single proton. That tells us nothing about the nuclear force, but the lack of diprotons and dineutrons does. Like Neil Spooner said in his physics 303 course, there are no proton-proton or neutron-neutron bound states. However a proton will bind with a neutron to form the 1875.6 MeV deuteron. With a relatively low binding energy of 2.224 MeV, the deuteron is said to have a prolate disposition and a cloverleaf electric quadrupole moment. See the Wikipedia deuterium article for the magnetic dipole moment: “the measured value of the deuterium magnetic dipole moment is 0.857 µN which is 97.5% of the 0.879 µN value obtained by simply adding moments of the proton and neutron”. People tend to say that this means there’s not much orbital angular momentum, but the Wikipedia article also says any orbital angular momentum gives a lower binding energy, “primarily due to increasing distance of the particles in the steep gradient of the nuclear force”. The article goes on to say the deuteron is a superposition of the s=1 l=0 state and the s=1 l=2 state. The s=1 refers to the spin ½ proton sitting above the spin ½ neutron like the top-to-tail magnets. The l=2 refers to a cloverleaf-style orbital angular momentum. The article does go on to say “the first component is much bigger”, but doesn’t explain the impact of this. In quantum mechanics for engineers Leon van Dommelen says that even for an l=2 probability of only 5.8%, a “twilight effect” can be quite large. He also says “the root-mean square radial position of the nucleons away from the center of the nucleus should be about 1.955 fm”. He refers to the asymptotic S-state amplitude and the root mean square radius of the deuteron by J P McTavish dating from 1982. In addition van Dommelen says the Heisenberg uncertainty relationship implies that the kinetic energy of the deuteron must be at least 6.2 MeV, and that this “reflects the fact that the deuteron is only weakly bound”. Since that kinetic energy has to be rotational, here’s a picture:
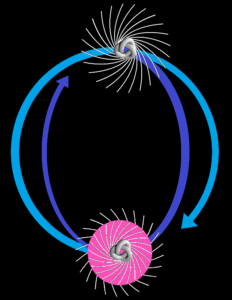 Based on a GNUFDL image by Ylebru
Based on a GNUFDL image by Ylebru
It isn’t a perfect picture, because it’s the wave nature of matter, not the pinwheel nature of matter. Those trefoils should be “inflated” so much they should look spherical, like an electron in its s-orbital. Moreover the nucleons don’t have surfaces, and they don’t go around each other like neutron stars. The electron in an orbital is a spherical spin ½ standing s-wave that can be distorted into a figure-of-eight p-wave disposition, and the same applies to neutrons. And protons. But nevertheless I like to think it’s a better picture, because as Neil Spooner said in his Sheffield physics 303 course: the nuclear force must apply a torque. We are dealing with spinors, not point particles. Charged particles move the way that they do because of torque. They’re all torque. So this picture will have to do for now until I can come up with a better picture, or better still find an animator. Until then the moot point is that the proton magnetic moment in terms of nuclear magnetons is 2.792 μN whilst the neutron magnetic moment is −1.913 μN. Those magnetic moments are not equal and opposite, so nor are the partners in the deuteron dance. That 2.224 MeV binding energy isn’t low for nothing.
Deuteron binding energy and kinetic energy
Talking of binding energy, I’d like to make it clear that whilst binding energy is sometimes portrayed as negative energy, it’s really less energy. When two charged particles are attracted towards one another, some of their internal kinetic energy aka potential energy or mass-energy is converted into external kinetic energy. When they meet, this kinetic energy is typically radiated away, leaving the particles with less mass-energy. Hence the mass deficit. We need to add this energy back to separate the particles, pulling at their mutual attraction rather like stretching a spring. However as Thayer Watkins points out on his applet magic website, an electron absorbed by an ion might lose 27.2 eV of potential energy, of which the binding energy is only 13.6 eV. That’s how much goes into the emission of a photon. The other 13.6 eV is retained as electron kinetic energy within the atom. For the deuteron the binding energy / kinetic split is not 50:50, and that’s why the deuteron is weakly bound. However whilst it’s weakly bound, the 2.224 MeV binding energy is more than four electrons’ worth, and springs don’t stretch on their own. In similar vein deuterons don’t decay into two protons and an electron. There isn’t enough energy for that. A proton weighs in at 938.272 MeV, two protons weigh in at 1876.544 MeV. Even without the electron and neutrino, the deuteron mass of 1875.6 MeV means we’re a million electron volts short. Hence the deuteron does not decay. However this is not the case for hydrogen-3, which is commonly known as tritium.
The triton and the helion
The tritium nucleus or triton can decay, just as a slightly-stretched spring can contract. It has a half-life of 12 years. It’s comprised of one proton and two neutrons, and has a magnetic moment of 2.978 μN. That’s similar to the proton magnetic moment of 2.792 μN which suggests the neutron spins largely cancel one another. The Wikipedia nuclear magnetic resonance article says tritium must have a pair of anti-parallel spin neutrons with a total spin of zero. However you might say the neutrons lend a little weight to the magnetic moment, since the triton magnetic moment is some 6.6% more than the proton magnetic moment. The triton has a relatively large binding energy of 8.4818 MeV – adding one neutron to a deuteron more than doubles the binding energy per nucleon. However this extra binding energy does not confer stability. With a mass of 2808.921 MeV the triton isn’t quite stable because the helion or helium-3 nucleus has a mass of 2808.391 MeV. Take away 0.511 MeV for the beta-decay electron, and the triton can undergo a very weak 18 keV beta decay to become a stable helion comprised of two protons and one neutron. This helion or helium-3 nucleus has a binding energy of 7.7180 MeV and a magnetic moment of -2.127 μN. That’s similar to the neutron magnetic moment of -1.913 μN, which suggests the proton spins largely cancel one another. However you might say the protons lend a little weight to the magnetic moment, since the helion magnetic moment is some 11% more than the neutron magnetic moment. Helium-3 is unusual in that it’s the only nuclide comprised of protons and neutrons where there’s more protons than neutrons. That’s saying something. As for what the triton and the helion “look” like, here’s another picture:
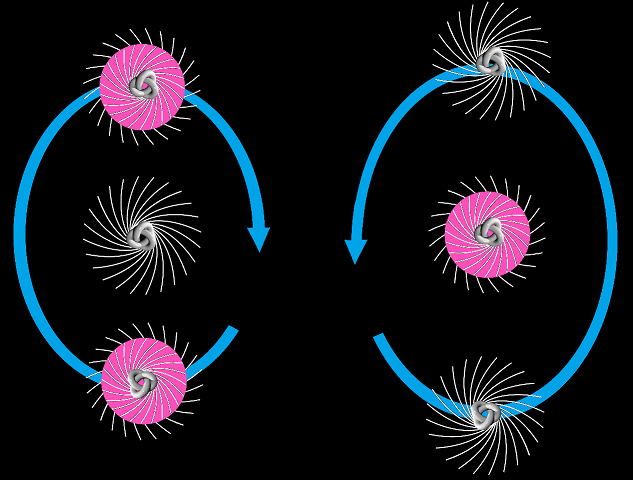 Triton and hellion, based on a GNUFDL image by Ylebru
Triton and hellion, based on a GNUFDL image by Ylebru
Again this isn’t a perfect picture, but I hope it gives some kind of improved concept. Note that an electromagnetic nuclear force would suggest the triton and the helion exhibit a linear arrangement rather than a triangular arrangement. This is not the case for helium-4.
The alpha particle
The helium-4 nucleus is very stable. The protons and neutrons are so well bound they’re often emitted as a unit, an alpha particle. The binding energy of 28.295 MeV is more than 12 times that of the deuteron, 3.33 times that of the triton, and 3.66 times that of the helion. Adding one neutron to a helion more than doubles the binding energy per nucleon. The result has no magnetic moment, and no net spin. It exhibits bosonic characteristics, because all the nucleon spins are equal and opposite. The alpha particle is not some “bideuteron” spinor dance, it’s more like a group hug. As for the configuration, we tend to think of the alpha particle as having a tetrahedral disposition, which suggests an efficient packing wherein the nucleons are closer together and binding energy is higher. Linus Pauling wrote about crystal packing in 1929 and came up with a close-packed spheron model for atomic nuclei in 1965. The Wikipedia nuclear binding energy article says physicists used to refer to a packing fraction calculation. In the Wikipedia atomic nucleus article you can read how packing protons and neutrons is like packing hard spheres. I think this is broadly correct, but with rules that say protons bind to neutrons and stay away from protons and vice versa. That would suggest the alpha particle is more of a diamond than a tetrahedron. The two protons exhibit a mutual repulsion, and so do the two neutrons. On top of that as Chadwick said there’s a saturation ”analogous to the saturation of valency bindings between two atoms, when each neutron is bound to two protons and each proton to two neutrons”. Hence the mass-5 roadblock. Helium-5 is spectacularly unstable, with a half-life of circa 7 x 10-22 seconds. It emits a neutron and turns into helium-4. You might think two protons and three neutrons would make for a stable nucleus, but it doesn’t. You cannot insert a neutron into the foursome, because that would leave you with two adjacent neutrons. If you try to tack a neutron onto one of the protons, it just won’t stick and is flung away by the nuclear torque. Four’s company and five’s a crowd when the spinors are balanced in perfect harmony like a well-oiled machine:
Is this a perfect picture? No, because spinors are three-dimensional, not flat. And because they’re waves going around and around, not cogs. But taking the idea for a spin around the simple nuclides works. It flies. Because there’s something here that hits the spot. Something evocative of our wheels within wheels world where everything spins. Something that makes it a better picture.
The nuclear force is electromagnetic
I am reminded again of what William Kingdon Clifford said in his space theory of matter. Nothing else takes place. Those waves in space are electromagnetic waves, and we use pair production to turn them into spin ½ particles. There are neutrinos too, but neutrinos are more like photons than electrons, and electrons and protons and neutrons have charge, even when it’s net zero charge. They move the way that they do because of the screw nature of electromagnetism, be it in a cyclotron or because of their close proximity to each other. In the latter situation the result is patterns, and more complex things. We can understand those things by exploring the evidence of decay chains and more, but on the way we have to say goodbye to other things. Things we have never seen, for which there is no evidence, even after fifty years. Things like quarks. Things that do not exist, like gluons, because the gluons in ordinary hadrons are virtual. They only exist in the mathematics of the model. Despite what people say, there is no evidence for them, or for quarks, but there is ample evidence for something else:
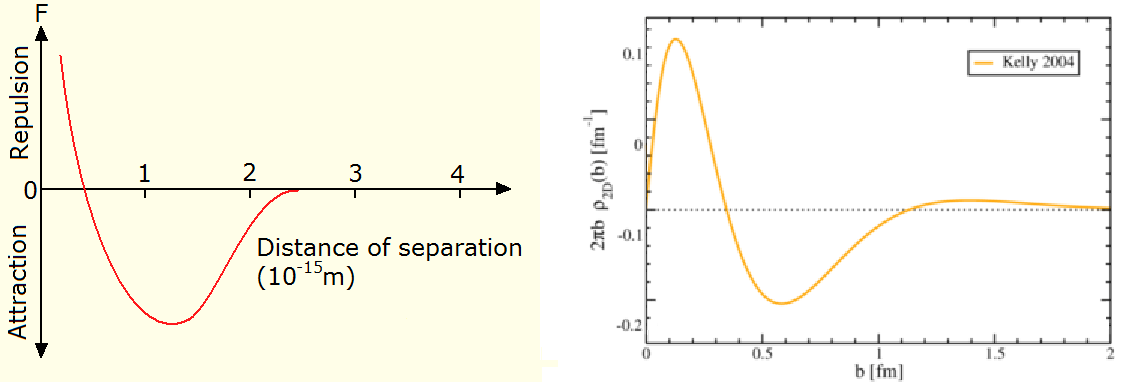 Nuclear force plot from the Dux college HSC physics course, neutron charge distribution image by Dru Renner, inverted by me
Nuclear force plot from the Dux college HSC physics course, neutron charge distribution image by Dru Renner, inverted by me
The more you learn the easier it is to see what it’s evidence for, and then it gets easier to find more evidence. It’s had no publicity, so it’s like finding buried treasure. Or the lost secrets of the ancients. Finding what Bernard Schaeffer has been saying for years is like finding AS carved on the wall of the cavern. Bernard Schaeffer is my Arne Saknussemm. What’s he’s saying is something that I think is blatantly patently obvious once you see it: the nuclear force is electromagnetic. Which means the strong force doesn’t exist.
Hi John, just a small point of similarity that the discussion of lost energy negative energy and binding energy puts me in mind of. When you first look at the equation E=mc^2 you might wonder where it comes from. In wondering that you might observe the kinetic energy of anything turns out to be 0.5mv^2. 1/2mv^2. Since v is velocity then for something with a velocity of c, the speed of light would have a kinetic energy of 0.5mc^2. But Einstein said it was double that. Now if your mass had reduced to near zero and all of your energy was kinetic then that is one thing. But the energy in E=mc^2 is double what you might expect from something with mass. What if to get to the higher speed all of your mass has to become kinetic energy? What if to get to the speed of light you need all of the energy you had wrapped up in denser forms released as kinetic energy moving as a displacement current at near maximum light speed? In my imagination light and mass are just electromagnetism in different forms. Moving in a straight line or a long curve light has little or no practically observable mass. Spin it in tight circles as electrons and the mass becomes visible. Combine the electrons to form protons and electrons with protons to form neutrons and you have the major forms of electromagnetism.I think it is a truly beautiful idea that there is a fundamental something that all things are made of. However, there are still practical issues to sort out. I think this is a really great blog. It is a treasure trove of solid research and insights, packed with useful references. As I have said before, keep up the good work John.
Many thanks Andy. The ½v² in the kinetic energy expression KE=½mv² is there because kinetic energy is telling you about the stopping distance when you apply a constant braking force. The stopping distance is the integral of the reducing velocity v. Or if you prefer, ½v² is the integral of all the velocities between 0 and v. When then you replace v with c, you can see how there’s a c² relationshiop between E and m. As for why it isn’t E=½mc², I’ve never thought about that. But all your energy is kinetic. See this paragraph from the mystery of mass is a myth:
.
In Compton scattering some of the incident photon energy is converted into electron kinetic energy. The electron gets kicked aside and the photon is deflected. The photon changes direction and its wavelength increases because it loses energy. The electron is accelerated in the usual sense and the photon is decelerated in the vector sense. Do another Compton scatter on this self-same photon, and another and another ad infinitum, and in the limit there’s no photon left. You can take all the kinetic energy away from a fast-moving cannonball, and you’re still left with a cannonball. But it isn’t the same for a wave. When you take all the kinetic energy away from a wave in the surf, it just isn’t there any more. So the wave is energy. It’s the same for a photon. When you take all the kinetic energy away from a wave in space, it just isn’t there any more. So the photon is kinetic energy. Perform Compton scattering repeatedly with the self-same photon, and in the end it has been entirely converted into the kinetic energy of electrons. And yet, and yet: in pair production, you can make an electron and a positron out of a photon. So the electron is kinetic energy too. But now this kinetic energy is hidden. We call it mass-energy.
.
Yes, I too think light and matter are electromagnetism in different forms. Maybe I should say waves, because neutrinos aren’t electromagnetic in the normal sense. But maybe that’s splitting hairs, because I did say the nuclear force is electromagnetic.
Rutherford suggested chemical combinatorics for neutron and nuclei. He suggested, that neutron is relative to atomic nuclei. Simple combination of one positive and one negative particle. With this model he predicted neutron and deuteron. Even before isotopes were discovered.
Positive particle for Rutherford model must be proton. No other particle. As nucleus of 1H – protium, lightest hydrogen isotope is single proton.
But negative particle inside neutron and nuclei cannot be electron. Due to mass of neutron and due to spin. Rutherfod did not know particles other that electron.
Chadwick spoiled whole idea of Rutherford. That was one of most critical error in physics. Real tragedy.
We know, that negative particle should be heavier that electron and should have spin 0 or 1.
That is boson family.
Most light charged boson with zero spin is negative pion. Real particle. That is easy way to identify negative particle of Rutherford model.
NB. Real particle, not virtual inside neurton and nuclei.
NB2. Negative pion, not neutral pion, which is different particle with same name.
Negative pion + proton = neutron
Atomic nuclei consist of real negative pions and real protons. If amount of negative pions is too big, it decays to electron and emits antineutrino. Beta-decay.
That Rutherford-Kudan model. Rutherford was right. He only knew nothing about particle zoo. But role of proton as subneutron and subnuclei particle were identified by Rutherford correctly. Almost 100 years ago.
That not fermion-fermion motion. That fermion-boson motion. Different mechanics.
The proton-neutron model and based on it quark model were errors. Chadwick speculated.
Still chemical approaches to investigation works great on that neutron/nuclei level. Better, than physical.
Thank you, John, for considerations, which helped to understand that similar idea for electron (The Egon Marx model) does not work. Electron cannot be represented as composite of negative pion and electronic neutrino, as negative pion is particle, but neutrino is not particle but wave propagating.
So, thank you for helping to understand, that there is no place to chemical approaches in study of electron, as that is purely physical object.
Reactions of composite neutron and nuclei are similar to chemistry.
But beta-decay is physical process. Transformation of one elementary particle (negative pion) into another elementary particle (electron) with propagation of spin wave (neutrino).
The only question is not clear to me.
Why fermionic proton gave compact nuclei with bosonic negative pion, but do not ‘like’ fermionic electron at small distances…
That is obviosly not matter of mass of negative particle, as change of electron to muon, which is almost as heavy as negative pion, changes nothing…
But that is purely physical effect, I can hardly find solution…
Rutherford was a smart guy. Yes, the negative particle inside the neutron can be an electron. It isn’t called electron capture for nothing. Beta particles are electrons. The neutrinos and antineutrinos complicate matters, but remember the neutron’s charge is negative on the outside and positive on the inside. That’s because it’s an electron twisted and mangled into a proton. It wasn’t Chadwick who spoiled Rutherford’s idea. Chadwick said “the electric field between a neutron and a nucleus is small except at distances of the order of 10ˉ¹² cm”. Chadwick didn’t say pion field or strong-force field, he said electric field.
.
I’m sorry Pavel, but a neutron is a not a proton plus a negative pion. Atomic nuclei do not consist of real negative pions and real protons. Beta decay occurs for a free neutron, and we don’t see pions. We don’t see quarks either.
.
No, the electron cannot be represented as a composite particle made up of a negative pion and an electron neutrino.
.
Fermionic protons form compact nuclei with neutrons. And neutrons are like close-couple hydrogen atoms, where the electron has been partially pulled into the proton. But some of its negative charge stays on the outside, so a neutron can bind two protons together. See the last picture in the article. The nuclear force matches the neutron charge distribution.
That was Chadwick to manifest that neutron is particle.
Rutherford’s idea that neutron is composite (of particles) – 1 positive particle and one negative. The same for nuclei. Rutherford also proved that positive particle should be proton. As he knew only one negative particle – electron, it was his first suggestion.
You talk about neutral (at average) neutrons binding positive protons.
Rutherford told about positive protons binded by negative particles. Much much more EM interaction, not?
You talk about transmutation of elementary particles. Proton field + electron field + twisting = complitly new elementary particle neutron.
Rutherford told that neutron is similar to atom. 1 positive 1 negative particles. And nucleus is similar to atoms several positive several negative, but amount of positive greater.
Without twist (transmutation of particles) electron can not be partner of proton in Rutherford model of composite neutron and nuclei. Twisting of field is tool for transmutation, convertion between elementary particles, it changes particles.
Positive particle in that model was discovered by Rutherford, so known to Rutherford and correctly identified by Rutherford. It really may be only proton.
Negative particle for Rutherford cannot be electron – only negative particle known to Rutherford.
Chadwick closed the Rutherford model too early.
As we may identify that negative particle. Negative pion explains observable phenomenon and properties well, at least, basic.
That Rutherford-Kudan model.
Positive fermionic protons and negative bosonic pions together creating both neutron and nuclei by binding.
No reason to think how neutral particle may bind to positive any more.
But reason to think about difference in dynamics of charged fermions and charged bosons, as quantum mechanics was focused on fermions only yet.
Chadwick = proton-neutron model (neutron is elementary)
Rutherford = proton-negative particle model (neutron is composite of particles, just particular case).
If we say about reaction proton+electron+twist = neutron (elementary), and neutrons bind protons, that is sophisticated variant of the proton-neutron model. Not Rutherford model.
As in Rutherford negative particles are just in collective motion with positive. Not captured/destroyed to give new elementary particle by transmutation/convertion.
I understood, John, thank you for that.
To speak about composite particles and elementary is one of roots of misconception in physics.
Correctly would be to speak about elementary particles and composites of particles.
For example, molecules are composite of atoms. Both may be considered as particles. But not correct to mix.
On neutron the question is if neutron is particle or composite of particles.
Would not you like to confuse molecules (which are particles) with atoms (which are particles too) with protons and electrons (which are particles) with propagation of waves in space (photon, neutrino)?
Elementary particles, composites of elementary particles and waves propagating would be three correct categories for that.
The difference is important to feel to understand. Inside composite of particles, each particle conserves its identity. They just binded. Collective dynamics.
On opposite, elementary particles. They may transform (transmutate, coverts) into other elementary particles, but in that case they will loose identity.
Say, you may have ball made of copper binded to ball made of tin (composite of balls). One body, made of two.
Or you can fuse them into one ball. You will get a piece of bronse (new ball).
Different situations.
First is for elementary particles binded into composite of particles.
Second is for transmutation of two elementary particles into other elementary particle. There are no tin separate and no copper separate in bronse. New material. Identity loosed.
Proton + electron = neutron after twist of field of electron and proton is such fusing into new elementary particle? Or I did not understand concept of twist?
Prof Prem raj Pushpakaran writes — 2019 marks the 100th year of the discovery of positively charged stable subatomic particle, Proton!!!
Sadly Prof, here we are a hundred years later and particle physicists still don’t know what a proton is.
Thanks again. I agree that the strong force does not exist. Another clue is the weird almost equal strengths of EM and string forces. If they are are so different then how come they have about the same force? The current dogma in physics is that coupling constants are basically unrelated, so the strong force might have been 1000 or a million times stronger or weaker, ruling out atoms, etc.
Also try and find the strong force acting on its own, with no EM around.
Good point The question I like to ask is where does the strong force go in low-energy proton-antiproton annihilation to gamma photons? Poof! It’s gone, in a flash. Along with all those quarks and gluons. Which we have never actually seen. Yes, particle physics is just dripping with dogma. And not just QCD. PS: Your website looks interesting.
While searching for information online, I came across this website, and I find this kind of discussion to be quite excellent.
The nuclear force is essentially the interaction of magnetic moments between nucleons. The issue lies in the formula for the magnetic moment interaction energy in electrodynamics, which does not account for the strong magnetic flux coupling that occurs at close distances, similar to what is observed in transformers and toroidal coils. This strong magnetic flux coupling can allow the nuclear binding energy between protons and neutrons to reach up to 125 MeV, far exceeding the average specific binding energy of 8 MeV in atomic nuclei.
Moreover, the nuclear repulsion force is essentially Lenz’s impedance, a type of non-conservative force that can sometimes manifest as an attractive force. Within the deuteron, protons and neutrons are in a state where their magnetic moments attract each other, while simultaneously inducing additional magnetic moments that generate a repulsive force, resulting in a state of relative rest. This is why the deuteron does not exhibit discrete nuclear spectra. The nucleons inside the atomic nucleus are somewhat akin to being in a state of superconducting magnetic levitation.
The meson field theory and quantum chromodynamics find themselves in a somewhat awkward position. Additionally, with the inclusion of Lenz’s impedance, a non-conservative force, the uncertainty principle no longer holds. The uncertainty principle is a characteristic of conservative fields and is not a fundamental property of quantum systems. Classical mechanics, if only conservative forces are considered, would also exhibit uncertainty properties.
Incorrect magnetic moment interaction formula:
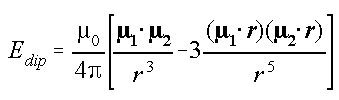
Article available at:
https://figshare.com/authors/CAO_Zexin_/15182149
1, Is the nuclear force just magnetic moment interaction between nucleons?
2, Nucleon-nucleon repulsion might come from Lenz’s law
Interesting stuff, Cao. I will take a look. Meanwhile, note that there are no diprotons or dineutrons. Neutrons don’t stick to neutrons, and protons don’t stick to protons. Which means the nuclear force is not a force between nucleons. It’s a force between protons and neutrons.
You’re right. Nuclear forces mainly occur between protons and neutrons. In my article, I also focus mostly on the interactions between protons and neutrons, particularly considering the Lenz impedance of neutrons. However, the differing binding energies of helium-3 and tritium nuclei indicate that the interactions between nucleons are not one-to-one. Additionally, dineutron nuclides do exist; some atomic nuclei emit dineutron nuclides, although their lifespan is exceedingly brief, lasting only about 10^{-22} seconds. Furthermore, physicists have synthesized extremely short-lived tetraneutron atoms, which suggests that there is some interaction between two neutrons as well.
.
The Coulomb repulsion between protons is too strong, and our calculations demonstrate that diproton nuclei, even when including magnetic moment interactions, cannot satisfy the equilibrium conditions.
Excellent paper.
I guess that animation of photon and eventually electron that satisfies all that been said on your site (PD) will be sort of first look on real picture of our world’s fathers. Still, we may dream about it.
It’s probably not that difficult, Tony. I did see a photon animation once, in a grid. It looked something like the oceanic swell waves I saw when we were on a cruise a few years back. They were a little like this:
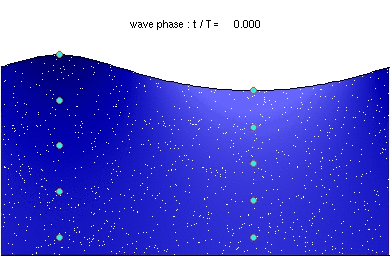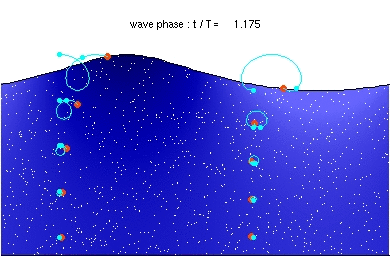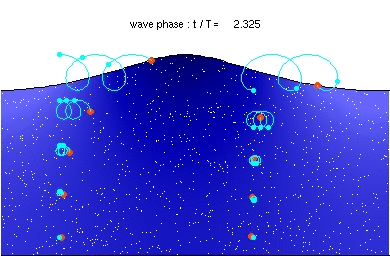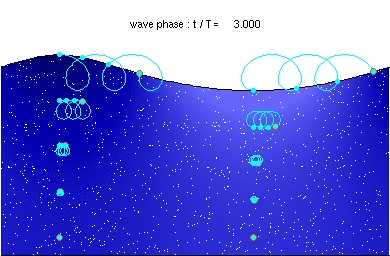
However space doesn’t have a surface. So the first step is to take two oceans like the above, turn one upside down, and plonk it down on top of the other. Try to imagine you’re a diver, deep in a well-lit crystal clear sea, and a wave comes at you. You would only see it if you saw fish etc moving en masse in a circling motion that got closer and closer. A photon isn’t the same as a wave in the ocean, but the wave in the ocean is a start.
https://www.popularmechanics.com/military/aviation/a34716703/navy-detect-submarines-with-airborne-radar-p8-poseidon/
.
My old Job
.
Assume space is incompressible!
Steve, I love the continuous path that microwave technology has achieved. Yea ,the ideas of smaller, more powerful, micro pixelated active & passive arrays is quite old in theory, but obviously had to wait for materials and technologies to advance.
Even deep underwater, it’s hard to outwit the Laws of Thermodynamics. Methinks that prime suspects would be bubble wakes and natural static/ion and of possible flows/discharges ?
And a way to transcribe sonic noises via said EM arrays ?
Lots of microphones over a large area for sonar.
Mucrowaves go direct to A/D converter instead of heterodyne. This was 30 years ago.
There was a sono buoy that dropped to the deep bottom, anchored, payed out a balloon which raised a glider which flew some distance away to get a triangulation
.
Sci fi stuff was way over my pay grade.
I swam besides these things and prepped them before they sank for deep testing
True story, in the 1980s we had an un-baffeled heated waterbed . In the winter when the relative humidy was extremely low, pushing 🫸 hard at the far end would generate a large visual wave. What our naked human eyes can’t see is the EM heat way produced in the infrared spectrum. One strong enough to turn on or off our small bedroom, parents only size t.v. set !
Greg: did I understand that right? When you gave your waterbed a shove, it turned on the TV?
Yes indeed. And turned it off as well !
The bed lacked internal baffles and my ex-wife (like most females is cold blooded) kept the heater thermostat turned up full blast in the winter. Filled with a naturally occurring high iron/mineral content of local tap water and combined with low air humidity : causing
a mostly broad spectrum infrared wave produced by physical wave first is my best, educated hypothesis.
American t.v./ hi-fi remotes still work on the infrared spectrum you know.
Greg: patent it! Quick!
I don’t have the ways or means Boss, therefore anyone may try themselves. Anyways, for under a $10.00 greenback , I can purchase a modern pre-coded universal remote about anywhere.
I do encourage others to replicate with a small, grade school level science experiment.
And teach some Karl Popper 101 at the same time.
PD; Almost first of the month, I find myself checking your site more frequently. Just realized PD. Can stand for Pavlov’s dog!.
Steve, not too long ago I fell down the Dr. Pavlov & co-horts rabbitt hole on Wikipedia.
It’s right out of H.P. Lovecraft’s playbook and eriely predates the horrors of the Nazi Holocaust .
For your amusement:
.
https://en.wikipedia.org/wiki/Greisen%E2%80%93Zatsepin%E2%80%93Kuzmin_limit
.
https://en.wikipedia.org/wiki/List_of_unsolved_problems_in_astronomy