There’s some ambiguity when it comes to quantum mechanics. Some people apply the term widely, others apply it to the theory that was developed in the 1920s to replace the old quantum theory. There’s some ambiguity with that too, in that the old quantum theory was primarily an atomic model proposed by Niels Bohr in 1913 and extended by Arnold Sommerfeld in 1916. It didn’t include the quantum nature of light, which arguably began with Max Planck’s black-body paper in 1900. Or with Albert Einstein’s photoelectric paper in 1905. Bohr didn’t agree with the quantum nature of light, and was still arguing about it in the early 1920s. He was proven wrong, and the old quantum theory was replaced by a new quantum theory which included aspects of an even older quantum theory. As for what it’s called, Max Born called it quantum mechanics, so that will do for me.
Compton scattering
As for when this quantum mechanics began, it’s hard to say. Wolfgang Pauli was talking about the Bohr magneton in 1920. Arthur Compton was talking about electron spin in his paper the magnetic electron in 1921. He referred to the Parson electron or magneton which featured a rotation with a “peripheral velocity of the order of that of light”. Compton said “we may suppose with Nicholson that instead of being a ring of electricity, the electron has a more nearly isotropic form”. The Stern-Gerlach experiment was performed in 1922. It demonstrates that “particles possess an intrinsic angular momentum that is closely analogous to the angular momentum of a classically spinning object, but that takes only certain quantized values”. 1922 was also when Arthur Compton discovered what’s now known as Compton scattering. Peter Debye was doing similar work at much the same time, so it’s sometimes referred to as the Compton-Debye effect. But see Compton’s 1923 paper A Quantum Theory of the Scattering of X-rays by Light Elements. The scattered X-rays had an increased wavelength, and the increase in the wavelength depended on the scattering angle. The results matched a model wherein one light quantum interacted with one electron:
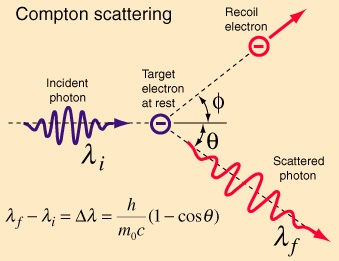 Image from Rod Nave’s hyperphysics
Image from Rod Nave’s hyperphysics
Compton had demonstrated the quantum nature of light. See his Nobel lecture for a nice slice of history. He described how X-rays were once thought to be comprised of “streams of little bullets called ‘neutrons’”, and weren’t thought to be a form of light. But then he referred to Barkla and Stenström and others, and said “it would take a bold man indeed to suggest, in light of these experiments, that they differ in nature from ordinary light”. X-rays were light. But “to account for the change in wavelength of the scattered rays, however, we have had to adopt a wholly different picture of the scattering process, as shown in Fig. 9. Here we do not think of the X-rays as waves but as light corpuscles, quanta, or, as we may call them, photons”. Light has a wavelength, but emission is directed. The energy of light is discontinuously distributed in space, as energy quanta which move without dividing. Einstein was right. Light consists of photons.
The wave nature of matter
Another important step took place in 1923 when Louis de Broglie sent a letter to Nature on waves and quanta. He said he’d ”been able to show that the stability conditions of the trajectories in Bohr’s atom express that the wave is tuned with the length of the closed path”. His thesis followed in 1924. See the English translation by Al Kracklauer. It’s on the theory of quanta, and it includes a useful historical survey. De Broglie referred to Christiaan Huygens and his undulatory theory of light, and to Isaac Newton and his corpuscular theory of light. He also said when Augustin-Jean Fresnel developed his “beautiful” elastic theory of light propagation, Newton’s ideas lost credibility. De Broglie then talked about Max Planck and the energy exchange between resonator and radiation taking place in integer multiples of hν. He talked about Einstein and the photoelectric effect, saying Einstein instinctively understood that one must consider the corpuscular nature of light. He referred to his brother Maurice De Broglie along with Rutherford and Ellis and said photo-electric studies “have further substantiated the corpuscular nature of radiation”. But he also referred to von Laue, Debye, and W L Bragg and said “the wave picture can also point to successes”. He referred to Compton too, and said “the time appears to have arrived, to attempt to unify the corpuscular and undulatory approaches”.
 Bohr-de Broglie atom by Kenneth Snelson
Bohr-de Broglie atom by Kenneth Snelson
De Broglie said “the fundamental idea pertaining to quanta is the impossibility to consider an isolated quantity of energy without associating a particular frequency to it”. And that the phase-wave concept permits explanation of Einstein’s condition. He also said propagation is analogous to a liquid wave in a closed channel, wherein the length of the channel was resonant with the wave. And that this can be applied to the closed circular Bohr orbits in an atom. De Broglie was talking about corpuscles and phase waves, but saying “a corpuscle and its phase wave are not separate physical realities”. That doesn’t seem to square with what people say about pilot waves. However that’s perhaps because he “left the definitions of phase waves and the periodic phenomena for which such waves are a realization, as well as the notion of a photon, deliberately vague”. That’s a shame, but nevertheless he’d planted a seed.
The Pauli exclusion principle
Meanwhile in 1924 Wolfgang Pauli proposed a fourth quantum number to explain the anomalous Zeeman effect. See Wolfgang Pauli announces the exclusion principle written by Ernie Tretkoff in 2007. Pauli had spent a year in Copenhagen with Bohr, and later said “the question, as to why all electrons for an atom in its ground state were not bound in the innermost shell, had already been emphasized by Bohr as a fundamental problem in his earlier works”. Also see the Wikipedia Pauli exclusion principle article, which says he found an essential clue in a 1924 paper by Edmund Stoner. This led Pauli to realize that the numbers of electrons in closed shells can be simplified to one electron per state, provided electron states were defined using the three existing quantum numbers plus a new two-valued fourth state. In early 1925 Pauli wrote a paper on the connexion between the completion of electron groups in an atom with the complex structure of spectra. That’s where he said cases are excluded “where both electrons have m1 = ½ or both have m1 = -½; rather, we can only have m1 = ½ for the first electron and m1 = -½ for the second electron”. The Pauli exclusion principle was born. Pauli later said “physicists found it difficult to understand the exclusion principle, since no meaning in terms of a model was given”. But he also said the gap was filled by Uhlenbeck and Goudsmit’s idea of electron spin.
It means that the electron has a spin, that it rotates
There’s a reader-friendly article on the discovery of the electron spin where Samuel Goudsmit gives the history: “When the day came I had to tell Uhlenbeck about the Pauli principle – of course using my own quantum numbers – then he said to me: “But don’t you see what this implies? It means that there is a fourth degree of freedom for the electron. It means that the electron has a spin, that it rotates””. Goudsmit knew about the spectra, and said “if one now allows the electron to be magnetic with the appropriate magnetic moment, then one can understand all those complicated Zeeman-effects. They come out naturally, as well as the Landé formulae and everything, it works beautifully”.
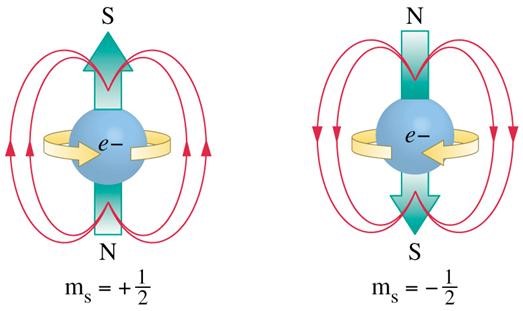 Image from Princeton modern understanding originally from General Chemistry 3rd edition, by Hill and Petrucci
Image from Princeton modern understanding originally from General Chemistry 3rd edition, by Hill and Petrucci
Goudsmit also said the man who never cared to believe in spin was Pauli. And that Llewellyn Thomas sent him a letter saying Ralph Kronig had had the idea a year previously. See the Wikipedia Ralph Kronig article for more. It says Kronig proposed electron spin in January 1925 after hearing Pauli in Tübingen, but Heisenberg and Pauli hated the idea, so Kronig didn’t publish. However Goudsmit and George Uhlenbeck didn’t stop to ask. Their paper was published in November 1925, and the spin ½ electron was born. Their subsequent paper on spinning electrons and the structure of spectra was printed in Nature. It was followed by a note from Niels Bohr who said this: “In my article expression was given to the view that these difficulties were inherently connected with the limited possibility of representing the stationary states of the atom by a mechanical model. The situation seems, however, to be somewhat altered by the introduction of the hypothesis of the spinning electron which, in spite of the incompleteness of the conclusions that can be derived from models, promises to be a very welcome supplement to our ideas of atomic structure”. Llewellyn Thomas was of course responsible for Thomas precession, see his April 1926 paper on the motion of the spinning electron.
Matrix mechanics
Meanwhile Werner Heisenberg was joining the fray. Like Pauli he was a Sommerfeld student, but unlike summa cum laude Pauli, he had angered experimentalist Wilhelm Wien and almost flunked his doctorate. See The Sad Story of Heisenberg’s Doctoral Oral Exam by David Cassidy. Also see the Wikipedia article on Heisenberg’s entryway to matrix mechanics along with the matrix mechanics article. After a seven-month stint with Bohr in Copenhagen, Heisenberg took note of Hendrik Kramer’s 1924 paper The Law of Dispersion and Bohr’s Theory of Spectra. Then he famously travelled from Göttingen to Helgoland in June 1925 to combat his hay fever, and came back in July with a paper called Quantum-Theoretical Re-interpretation of Kinematic and Mechanical Relations. In it Heisenberg said “it seems more reasonable to try to establish a theoretical quantum mechanics, analogous to classical mechanics, but in which only the relations between observable quantities occur”. His paper was described as magic, as if it was some mathematical retrofit. It offered no description of what was going on inside the hydrogen atom, and it didn’t refer to de Broglie at all. In addition, whilst it’s usually described as Heisenberg’s matrix mechanics paper, it “neither uses nor even mentions matrices”. That was down to Max Born, who recognised the underlying formalism, and who wrote a paper on quantum mechanics with his assistant Pascual Jordan.
Five of the seven ideas were Jordan’s
There’s an English translation courtesy of David Delphenich, and an abridged version in Bartel van der Waerden’s 1967 book Sources of Quantum Mechanics. The latter is online courtesy of the internet archive, and contains a significant number of references to Fourier, plus some useful history – Pauli was cold and sarcastic, and five out of seven ideas were Jordan’s. Two months later in November 1925, Born, Jordan, and Heisenberg finished their follow-up Dreimännerarbeit “three-man work” paper on quantum mechanics II. They expressed their conviction that difficulties could only be surmounted via a mathematical system which “would entirely consist of relations between quantities that are in principle observable”. And that such a system “would labour under the disadvantage of not being directly amenable to a geometrically visualizable interpretation”. They also said the motions of electrons could not be described in terms of the familiar concepts of space and time. Van der Waerden’s book also gives Pauli’s paper on the hydrogen spectrum from the standpoint of the new quantum mechanics. It says this showed that the hydrogen spectrum can be derived from the new theory, and that Pauli said “Heisenberg’s form of quantum theory completely avoids a mechanical-kinematic visualization of the motion of electrons in the stationary states of the atom”. As if it was a virtue.
Debye was right
It wasn’t just Heisenberg and co joining the fray, it was Dirac too, and Schrödinger. See Heisenberg and the early days of quantum mechanics by Felix Bloch. It’s a charming recount: “at the end of a colloquium I heard Debye saying something like: “Schrödinger, you are not working right now on very important problems anyway. Why don’t you tell us some time about that thesis of de Broglie, which seems to have attracted some attention”. So, in one of the next colloquia, Schrödinger gave a beautifully clear account of how de Broglie associated a wave with a particle and how he could obtain the quantization rules of Niels Bohr and Sommerfeld by demanding that an integer number of waves should be fitted along a stationary orbit. When he had finished, Debye casually remarked that he thought this way of talking was rather childish. As a student of Sommerfeld he had learned that, to deal properly with waves, one had to have a wave equation. It sounded quite trivial and did not seem to make a great impression, but Schrödinger evidently thought a bit more about the idea afterwards. Just a few weeks later he gave another talk in the colloquium which he started by saying: “My colleague Debye suggested that one should have a wave equation; well, I have found one!” And then he told us essentially what he was about to publish under the title “Quantization as Eigenvalue Problem” as a first paper of a series in the Annalen der Physik. I was still too green to really appreciate the significance of this talk, but from the general reaction of the audience I realized that something rather important had happened, and I need not tell you what the name of Schrödinger has meant from then on. Many years later, I reminded Debye of his remark about the wave equation; interestingly enough he claimed that he had forgotten about it and I am not quite sure whether this was not the subconscious suppression of his regret that he had not done it himself. In any event, he turned to me with a broad smile and said: “Well, wasn’t I right?”
Wave mechanics
He was. Erwin Schrödinger had a head start because he’d written a paper in 1922 on a remarkable property of the quantum orbits of a single electron. He had a heads-up too. See Foundations of Quantum Mechanics in the Light of New Technology where you can read Chen-Ning Yang’s 1997 paper Complex Phases in Quantum Mechanics. Yang said Einstein alerted Schrödinger to de Broglie’s thesis, and Schrödinger wrote back to Einstein in November 1925. Schrödinger said “the de Broglie interpretation of the quantum rules seems to me to be related in some ways to my note in the Zs. F. Phys. 12 13 1922 where a remarkable property of the Weyl ‘gauge factor’ exp[-∫ϕdx] along each quasiperiod is shown”. Schrödinger submitted the first part of his four-part paper in January 1926. It was called quantization as a problem of proper values, part I. He talked of the hydrogen atom and said integralness arises in the same natural way as the node numbers of a vibrating string. He also talked of the azimuthal quantum number and said “the splitting up of this number through a closer definition of the surface harmonic can be compared with the resolution of the azimuthal quantum number into an ‘equatorial’ and a ‘polar’ quantum’”. He said “It is, of course, strongly suggested that we should try to connect the function ψ with some vibration process within the atom”. And that he was led to these deliberations by the suggestive papers of M Louis de Broglie. But that the “main difference is that de Broglie thinks of progressive waves, while we are led to stationary proper vibrations”. Schrödinger was talking about spherical harmonics, standing waves, and atomic orbitals:
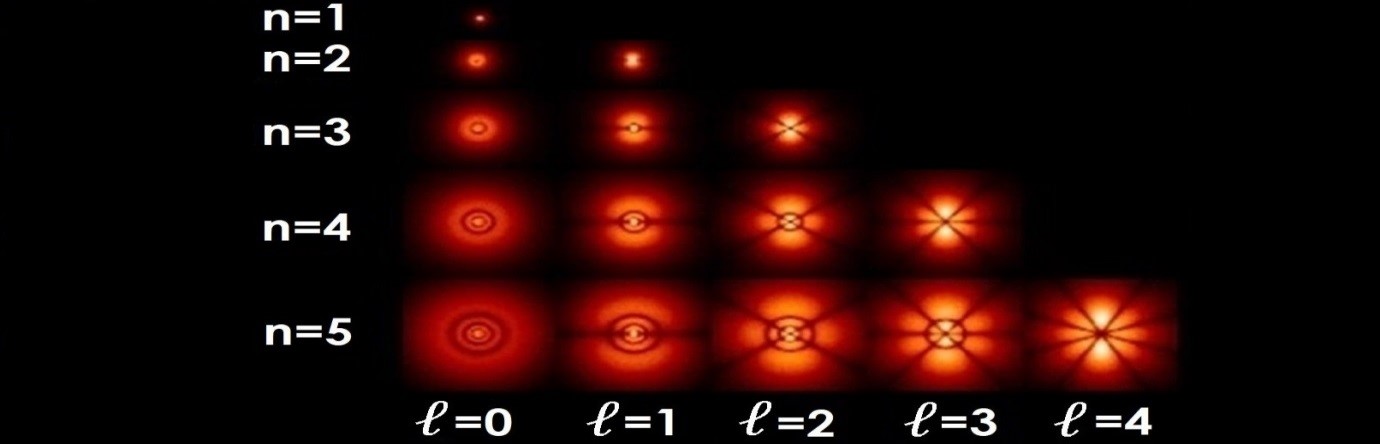 Image from The Star Garden article Sommerfeld’s atom by Dr Helen Klus
Image from The Star Garden article Sommerfeld’s atom by Dr Helen Klus
He also said “It is hardly necessary to emphasize how much more congenial it would be to imagine that at a quantum transition the energy changes from one form of vibration to another, than to think of a jumping electron”. His second paper quantization as a problem of proper values, part II was more of the same. He talked about wavefunction and phase and geometrical optics, and on page 18 said classical mechanics fails for very small dimensions of the path and for very great curvature. He talked of a wave system consisting of sine waves where the frequency works out to be ν=E/h, and said we can attempt of build up a wave group which will have relatively small dimensions in every dimension. On page 26 he said “let us think of a wave group of the nature described above, which in some way gets into a small closed ‘path’, whose dimensions are of the order of the wave length”. And that the wave group not only fills the whole path domain all at once but also stretches far beyond it in all directions. He said this: “All these assertions systematically contribute to the relinquishing of the ideas of “place of the electron” and “path of the electron”. If these ideas are not given up, contradictions remain. This contradiction has been so strongly felt that it has even been doubted that what goes on in the atom could ever be described within the scheme of space and time. From the philosophical standpoint, I would consider a conclusive decision in this sense as equivalent to complete surrender”.
Light rays show the most remarkable curvatures
He also said light rays “show, even in homogeneous media, the most remarkable curvatures, and obviously mutually influence one another”. That’s on page 27. I think it’s visionary stuff myself, though Schrödinger’s quantization as a problem of proper values, part 3 is arguably more mundane. It’s about perturbation theory and the Stark effect. But it does say this: “since then I have learned what is lacking from the from the most important publications of G E Uhlenbeck and S Goudsmit”. Schrödinger refers to the angular moment of the electron which gives it a magnetic moment, and says “the introduction of the paradoxical yet happy conception of the spinning electron will be able to master the disquieting difficulties which have latterly begun to accumulate”. But he also says that in the present paper the taking over of the idea is not yet attempted. He didn’t attempt it in his part 4 either. Or in the condensed English version in Physical Review. That was called An Undulatory Theory of the Mechanics of Atoms and Molecules. He ended up saying “The deficiency must be intimately connected with Uhlenbeck-Goudsmit’s theory of the spinning electron. But in what way the electron spin has to be taken into account in the present theory is yet unknown”. That’s why there is no spin in the Schrödinger equation. But no matter, there’s some great stuff in there. Like material points consist of, or are nothing but, wave-systems. What’s not to like? For Niels Bohr, plenty.
Schrödinger goes to Copenhagen
Take a look at page 192 of Walter Moore’s 1989 book Schrödinger, life and thought: “Schrödinger wanted to find the structure of such waves when they are refracted sufficiently to travel in one of the Bohr orbits”. Also see page 209. Schrödinger had sent Max Planck a preprint of his first paper, which Planck read “like an eager child”. Planck also showed it to Einstein, who wrote to Schrödinger in April 1926 saying “the idea of your work springs from pure genius”. Ten days later Einstein wrote again. He said “I am convinced that you have made a decisive advance with your formulation of the quantum condition, just as I am convinced that the Heisenberg-Born method is misleading”. By then Schrödinger had written a paper on the relation of the Heisenberg-Born-Jordan quantum mechanics to mine. He said “it is very strange that these two new theories agree with one another”. Along with “I refer in particular to the peculiar ‘half-integralness’ which arises in connection with the oscillator and rotator”. It comes across as civil and diplomatic, but there’s perhaps an undercurrent that says something like this is proper physics, not mysticism.
Their true feelings were not concealed
On page 221 Moore talks about the relationship between Schrödinger and Heisenberg. He says they were diplomatic in printed papers, but “in personal letters their true feelings were not concealed”. He quotes words like monstrous and abominable, and bullshit. He tells how Schrödinger was invited to lecture in Germany, and travelled to Stuttgart then Berlin where he stayed with the Plancks. And how the older generation of Berlin physicists such as Einstein, Laue, Nernst and Planck were impressed, so much so that “Planck began to consider seriously his plans to bring Schrödinger to Berlin as his successor”. Schrödinger then went to Jena thence Munich, where he repeated his Berlin lecture. Heisenberg was in the audience, and in the question-and-answer session he asked how Schrödinger ever hoped to explain the photoelectric effect and black-body radiation. But before Schrödinger could reply, “Willy Wien angrily broke in and, as Heisenberg reported to Pauli, ‘almost threw me out of the room’”. Moore says Heisenberg was upset and immediately wrote to Bohr. Whereupon Bohr wrote to Schrödinger to invite him to Copenhagen for some “serious discussions”. They did not go well. See page 222 of Manjit Kumar’s 2008 book Quantum: Einstein, Bohr and the Great Debate About the Nature of Reality. He’s referring to Heisenberg’s book The Part and the Whole. Bohr met Schrödinger at the station in late September 1926: “after the exchange of pleasantries, battle began almost at once, and according to Heisenberg, ‘continued daily from early morning until late at night’”. Bohr appeared even to Heisenberg to be a “remorseless fanatic, one who was not prepared to make the least concession or grant that he could ever be mistaken”. When Schrödinger took ill and took to bed, Bohr sat on the edge of the bed and continued the argument. The words Bohr and bully seem to go together.
The Copenhagen Interpretation
By then Max Born had written his paper on the quantum mechanics of collisions. He’d come up with what’s now known as the Born rule. On page 3 he said this: “If one translates this result into terms of particles, only one interpretation is possible. Φn,m(α, β, γ) gives the probability for the electron, arriving from the z-direction, to be thrown out into the direction designated by the angles α, β, γ, with the phase change δ”. He was saying the only possible interpretation is that the Schrödinger wave equation described probabilities rather than something that was actually there. This was the beginning of the Copenhagen interpretation. Andrew Zimmerman Jones gives an overview in his 2017 essay on The Copenhagen Interpretation of Quantum Mechanics. He says it’s a combination of Born’s probabilistic statistical interpretation, Heisenberg’s uncertainty principle, and Bohr’s concept of complementarity:
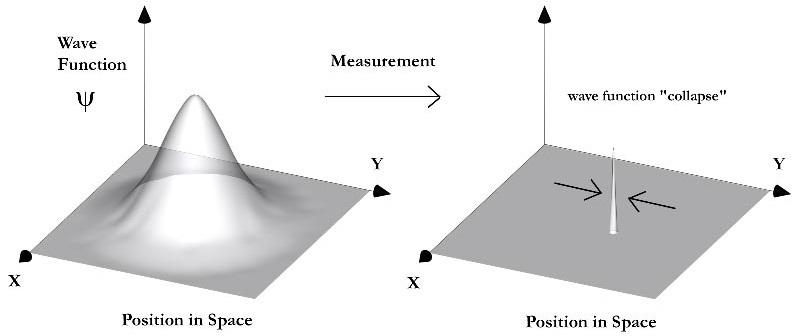 Copenhagen Interpretation image from Andrew Friedman’s website, see http://afriedman.org/
Copenhagen Interpretation image from Andrew Friedman’s website, see http://afriedman.org/
Heisenberg came up with his uncertainty principle in his March 1927 paper on the actual content of quantum theoretical kinematics and mechanics. He said “canonically conjugated variables can be determined simultaneously only with a characteristic uncertainty”. He also said the interpretation of quantum mechanics is still full of internal contradictions, “which become apparent in the battle of opinions on the theory of continuums and discontinuums, corpuscles and waves. This alone tempts us to believe that an interpretation of quantum mechanics is not going to be possible in the customary terms of kinematic and mechanical concepts”. Heisenberg was promoting the viewpoint he shared with Born, Jordan, and Bohr, even though his paper used the word “wave” 36 times. Hence the Wikipedia uncertainty principle article strikes a chord when it says this: “It has since become clearer, however, that the uncertainty principle is inherent in the properties of all wave-like systems, and that it arises in quantum mechanics simply due to the matter wave nature of all quantum objects”. What’s not clear however is why Heisenberg didn’t associate what came to be known as wavefunction collapse with the optical Fourier transform:
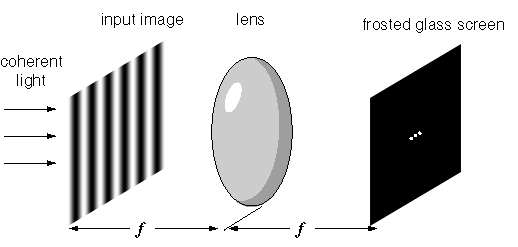 Image from Steven Lehar’s intuitive explanation of Fourier theory
Image from Steven Lehar’s intuitive explanation of Fourier theory
I should mention that he didn’t actually use the word collapse, he said at the instant of the determination of its position, the electron discontinuously changes its impulse. But the meaning is the same, and it’s closely related to Bohr’s complementarity. Heisenberg referred to that in an addendum to his paper, and Bohr talked about it in September 1927 in Como. You can find the details in his Nature paper on The Quantum Postulate and the Recent Development of Atomic Theory. I would say the message is “you can never hope to understand it”. Interestingly while all this was going on Pauli wrote a paper on the quantum mechanics of magnetic electrons. That’s where Pauli referred to Yakov Frenkel’s 1926 paper on the electrodynamics of rotating electrons, the one where Frenkel said the electron will thus be treated simply as a point. Pauli then wondered “whether such a formulation of the theory is even possible at all as long as one retains the idealization of the electron by an infinitely small magnetic dipole”. And “whether a more precise model of the electron is required for such a theory”. But he didn’t pursue it, more’s the pity. Because a few months later he could have talked about it at the Solvay conference.
The 1927 Solvay conference
The 1927 Solvay conference was given the title Electrons and Photons. But it wasn’t about electrons and photons at all. It was all about “the struggle between Einstein and the scientific realists” against “Bohr and the instrumentalists”. It is said that the latter won the argument. As to the truth of it, take a look at Quantum Theory at the Crossroads: Reconsidering the 1927 Solvay Conference written by Guido Bacciagaluppi and Antony Valentini in 2006. They give a good description of proceedings, including the reports by Bragg, Compton, de Broglie, Heisenberg and Born, and Schrödinger.
 Fifth Solvay conferance image, see Wikipedia
Fifth Solvay conferance image, see Wikipedia
They say “according to widespread historical folklore, the deep differences of opinion among the leading physicists of the day led to intense debates, which were satisfactorily resolved by Bohr and Heisenberg around the time of the 1927 Solvay meeting. But in fact, at the end of 1927, a significant number of the main participants (in particular de Broglie, Einstein, and Schrödinger) remained unconvinced, and the deep differences of opinion were never resolved”. They also say that “there has also been criticism – on the part of historians as well as physicists – of the tactics used by Bohr and others to propagate their views in the late 1920s”. That “a sense of unease lingers of the whole subject”. And that “’shut up and calculate’ emerged as the working rule among the vast majority”. See the Wikipedia article on the Einstein-Bohr debates for more. Bohr advocated a probabilistic quantum mechanics where there was no point trying to understand what was really going on inside the atom. Einstein said this meant quantum mechanics was incomplete, and that uncertainty was no substitute for understanding. Bohr defended his position by claiming that an independent reality in the ordinary physical sense can neither be ascribed to the phenomena nor to the agencies of observation. And that It is wrong to think the task of physics is to find out how nature is. Einstein said What we call science, has the sole purpose of determining what is. I agree one hundred percent with that.
The Copenhagen interpretation became mainstream orthodoxy
But amazingly, incredibly, the Copenhagen interpretation somehow prevailed. The Copenhagen interpretation became the mainstream orthodoxy. Despite the hard scientific facts. In 1917 the Einstein-de Haas effect demonstrated that spin angular momentum is indeed of the same nature as the angular momentum of rotating bodies as conceived in classical mechanics. In 1922 the Stern-Gerlach experiment demonstrated that the spatial orientation of angular momentum is quantized. In 1927 the Davisson-Germer experiment along with diffraction experiments by George Paget Thomson and Andrew Reid proved the wave nature of matter. In 1931 the Experimental proof of the spin of the photon was provided by Chandrasekhara Raman and Suri Bhagavantam. In 1932 Carl Anderson discovered the positron. In 1933 Patrick Blackett and Giuseppe Occhialini were the first to observe pair production. We could make electrons and positrons out of light, the electron had a different spin to the photon, and yet quantum mechanics surpasseth all human understanding? What happened? See the particle physics quantum theory timeline. It says in 1930 Max Born, after learning of the Dirac equation, said “physics as we know it will be over in six months”. In a way, a bad way, he was right.
What happened?
Paul Dirac made a significant contribution to quantum mechanics. See Kurt Gottfried’s 2010 essay P.A.M. Dirac and the Discovery of Quantum Mechanics for an overview. But note this on page 5: “Heisenberg, Dirac et al were hostile to wave mechanics because they thought it gave the misleading impression that the classical concepts of continuity and visualizability had survived the revolution, whereas they believed that it was a central virtue of their abstract theory that it did not evoke such delusions”. Perhaps that’s why Dirac’s 1927 paper on the physical interpretation of the quantum dynamics didn’t do what it said on the can. And why his 1928 paper the quantum theory of the electron doesn’t deliver a picture of the electron. The Dirac equation is said to describe the electron, but try explaining it to your grandmother, and you realise it doesn’t. Dirac wrote a paper in 1930 on the annihilation of electrons and protons, only they don’t. In a theory of electrons and protons he said negative kinetic energy appeared to have no physical meaning. That suggests he didn’t understand binding energy. He said the electron energy changes from positive to negative and energy of 2mc² is emitted. That suggests he didn’t understand E=mc². He said the Pauli exclusion principle prevents electrons decaying, and the “holes” in the distribution of negative-energy electrons are protons. That suggests he was talking out of his hat. As did his 1962 paper an extensible model of the electron, which depicted the electron as a charged conducting sphere with a surface tension. Yet in the Wikipedia timeline of quantum mechanics you can read that Dirac’s 1930 textbook Principles of Quantum Mechanics became a standard reference book that is still used today. Then Schrödinger’s cat, which illustrated the absurdity of the Copenhagen interpretation, was hijacked by the peddlers of mysticism to demonstrate just how “spooky” quantum physics is. Such people even advocate the many-worlds multiverse. What happened? More to the point, what didn’t? What didn’t happen at the 1927 Solvay conference was a discussion of what the photon was, or what the electron was. Then Bohr sold his pup to the world, and it was all downhill from there.
thx for dropping by to comment on my latest major effort. https://vzn1.wordpress.com/2018/05/25/fluid-paradigm-shift-2018/
am impressed youve already got some substantial dialogue going in your comments. would be very interested to hear from your readers also.
the quote by assistant/ acolyte heisenberg on his mentor bohr is really startling. “Bohr appeared even to Heisenberg to be a “remorseless fanatic, one who was not prepared to make the least concession or grant that he could ever be mistaken”. ” modern adherents/ acolytes of “shut up and calculate” are not much different than their cult leader. (using strong language here because hey, its the Duffield blog, wink)
its striking how some of physics history has been swept under the rug in the standard accounts bordering on dogma. this reminds me eg some of newton vs alchemy…
My pleasure vzn. Yes, there’s been some airbrushing of history going on. When it comes to gravity I’ve long known that people appeal to Einstein’s authority whilst flatly contradicting the guy. Then if you quote Einstein and refer to the digital papers, they’ll dismiss it as out of context or cherry picking. Now I also know that the same sort of thing has been going on in other sectors of physics. For example people peddle the mystery of wave/particle duality, but Pascual Jordan solved that mystery in 1925. The strong language is only there to show the enmity that existed back in the twenties, and which IMHO ended up causing some big problems.
.
PS: I started getting “adult” spam yesterday, so I turned comment moderation on. I really don’t like doing that, because I believe in free speech in science.
I think Schrödinger more or less nailed it with this:
.
“classical mechanics fails for very small dimensions of the path and for very great curvature”.
.
“let us think of a wave group… which in some way gets into a small closed ‘path’, whose dimensions are of the order of the wave length”.
.
“Light rays show, even in homogeneous media, the most remarkable curvatures, and obviously mutually influence one another”.
.
“it has even been doubted that what goes on in the atom could ever be described within the scheme of space and time. From the philosophical standpoint, I would consider a conclusive decision in this sense as equivalent to complete surrender”.
Bolches yarboclos, Batman!!!
What a useful post.
Thanks Poak. I hope everybody finds this and the other posts useful, When you dig into the history, your find some absolute treasure. Then you come to appreciate that some of the contemporary stuff is absolute junk.
I recently stumbled on your website (6/24) and have found it thought provoking. I had previously just read (from Infinity Puzzle, Frank Close) that what I’d been taught for years regarding EPR and Schrodinger’s cat was the exact opposite of what they were implying. Damn Bohr and his minions!
Welcome, Parish. I hope you will find that there’s a wealth of factual information here. Apologies for the links that don’t work. Link rot is the bane of my life. Let me know and I will try to fix any instances you find.