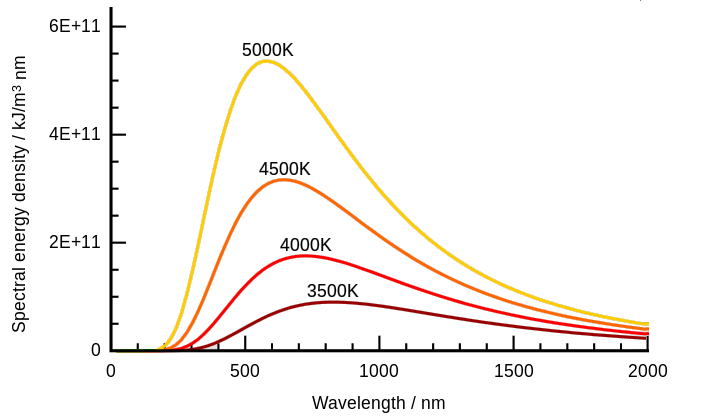
Quantum physics or quantum theory can arguably be traced back to Gustav Kirchhoff’s researches on the solar spectrum. See his 1860 paper on the relation between the radiating and absorbing powers of different bodies for light and heat. Kirchhoff spoke about black body radiation and what came to be known as the black body problem. When a body is heated such that it emits light, the colour of its glow depends on its temperature alone. As you heat a poker in a fire it glows cherry red, then orange, then yellow, and so on. Kirchhoff also said the overall radiation at various wavelengths is described by a universal function, and “the determination of this function is a problem of the highest importance”. See page 15 of his paper. He was talking about the black-body spectrum. There’s two aspects to it. A plot of emitted wavelengths at some temperature is a curve, and if you change the temperature you change the curve, as per Wilhelm Wien’s displacement law:
 Based on a CCASA image by 4C and other contributors, see Wikipedia commons
Based on a CCASA image by 4C and other contributors, see Wikipedia commons
See Kirchhoff’s law of thermal radiation on Wikipedia where you can read that Lord Rayleigh and Sir James Jeans tried to describe it in classical terms. They came up with the Rayleigh–Jeans law which started life in 1900. It was simple enough, based on standing waves in a cavity. You can fit more high-frequency waves in the cavity, and they have more energy. However it’s wrong, because what it predicts, is the ultraviolet catastrophe.
Max Planck and his constant of nature h
The derivation of Planck’s radiation law by Rhodri Evans gives some relevant history. He tells how the exact shape of the black body spectrum was determined by the Physikalisch-Technische Reichsanstalt (PTR) in Germany. By October 1900 Max Planck knew of experimental results from the PTR which showed that Wien’s distribution law didn’t fit the blackbody spectrum. Planck was a friend of Wien’s, and had filled Kirchhoff’s shoes as professor of physics in Berlin. He decided he’d try to find a mathematical curve to fit the data. After exhausting all other options, in “an act of desperation” he found an equation akin to Wien’s that worked:
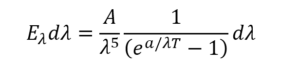
Planck didn’t know why it worked, but after six weeks he’d outlined a model that seemed to fit the bill. Evans summarises it nicely. The radiation was produced by oscillating electrons, and the total energy at some frequency was the energy of one oscillator multiplied by the number oscillating at that frequency. However Planck had to make some assumptions:
1) The energy of each oscillator wasn’t proportional to the square of the amplitude or frequency as it would be in classical physics, but to the frequency, E ∝ ν.
2) The energy of each oscillator could only be a multiple of some fundamental “chunk” of radiation hν, so En = nhν where n = 0, 1, 2, 3, 4 etc.
3) The number of oscillators of energy En was given by the Boltzmann distribution Nn = N0e¯nhν/kT where N0 is the number of oscillators in the lowest energy state.
Also see the black body radiation section of the Max Planck Wikipedia article. It refers to an article by Helge Kragh called Max Planck: the reluctant revolutionary. Kragh says the standard story featuring the ultraviolet catastrophe is a myth, and quantum theory “did not owe its origin to any failure of classical physics”. He also says Planck was guided by Boltzmann’s kinetic theory of gases, and formulated a principle of elementary disorder that didn’t rely on mechanics or electrodynamics. Planck came up with what’s now known as is Planck’s postulate and the Planck radiation law, introducing energy elements wherein the total energy of the black-body oscillators is divided into finite portions of energy via quantization. See Planck’s 1900 paper on the theory of the energy distribution law of the normal spectrum. That’s where Planck said this: “We consider, however – this is the most essential point of the whole calculation – E to be composed of a very definite number of equal parts and use thereto the constant of nature h = 6.55 ×10−27 erg · sec. This constant multiplied by the common frequency ν of the resonators gives us the energy element ε in erg, and dividing E by ε we get the number P of energy elements which must be divided over the N resonators”. Kragh later says quantum theory was born, but nobody noticed: “very few physicists expressed any interest in the justification of Planck’s formula, and during the first few years of the 20th century no one considered his results to conflict with the foundations of classical physics”. He also says Planck didn’t see his theory as a drastic departure from classical physics, and between 1901 and 1906 didn’t publish anything on black-body radiation or quantum theory. However in 1905, somebody else did.
Einstein says light is quantized
1905 was Einstein’s annus mirabilis. The first of his papers published in Annalen der Physik, where Planck was on the advisory board, was on a heuristic viewpoint concerning the production and transformation of light. Einstein was talking about the photoelectric effect:
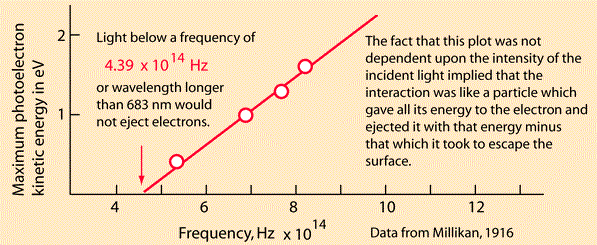 Image from Rod Naves’ hyperphysics
Image from Rod Naves’ hyperphysics
Einstein spoke of the distinction between the theoretical concepts of gases and bodies and the theory of electromagnetism. The former is determined by the positions and velocities of atoms and electrons, whilst the latter makes use of continuous spatial functions. He said the energy of a body “cannot be subdivided into arbitrarily many or arbitrarily small parts”, while the energy of a beam of light from a point source “is continuously spread an ever-increasing volume”. He said observations associated with blackbody radiation and other phenomena “are more readily understood if one assumes that the energy of light is discontinuously distributed in space”. He said light consists of energy quanta “which move without dividing, and which can only be produced and absorbed as complete units”. He talked about black body radiation emitted by electron oscillators, and referred to Planck’s paper and “a famous work by Herr W Wien”. He talked about gases and entropy and monochromatic radiation and Boltzmann’s principle, and about the emission of cathode rays through the illumination of solid bodies. That’s where he referred to Philipp Lenard’s “pioneering paper” and said energy quanta penetrate into the surface layer of the body, where their energy is transformed into the kinetic energy of electrons. Lenard had discovered a form of what was known as the Hertz effect in the ionisation of gases. He’d noticed that the energy of the emitted electrons increased with the frequency of the incident light as opposed to the intensity. See Philipp Lenard and the photoelectric effect by Bruce Wheaton for details. It dates from 1978. On page 320 you can read that “Einstein’s light quantum, which explained Lenard’s results at the cost of rejecting classical wave radiation, had little support early in the century”. So, what happened next? Not much.
Einstein says matter is quantized
Read John D Norton’s origins of quantum theory and you’ll see that there’s a gap between Einstein in 1905 and Niels Bohr in 1913. There isn’t much in the Wikipedia timeline of quantum mechanics article either. It’s similar in the particle adventure quantum theory timeline, which says Einstein was one of the few scientists to take Planck’s ideas seriously. Check out Einstein’s papers and you’ll notice that in 1906 he wrote a paper on the theory of light production and light absorption, and in 1907 he wrote a paper on Planck’s theory of radiation and specific heat. It is said that these papers showed that that both light and matter had a quantum nature, and E=hν was a general law rather than restricted to black body radiation. In 1909 Einstein wrote a paper on the present status of the radiation problem, and then a further paper on the development of our views on the composition and essence of radiation. The latter is described as a “pivotal address before the 81st assembly of the Gesellschaft Deutscher Naturforscher, held in Salzburg, where Einstein showed that photons must carry momentum and should be treated as particles”. It’s also where Einstein said the undulatory structure and the quantum structure “should not be seen as mutually incompatible”. He also said this:
 Excerpt from the development of our views on the composition and essence of radiation
Excerpt from the development of our views on the composition and essence of radiation
Einstein was almost the only one banging the quantum drum, it was important stuff, but it didn’t set the world on fire. Not immediately. There was what looked like contradictory evidence, such as George Taylor’s interference fringes with feeble light. There was also a lot going on. See volume 1 of the historical development of quantum theory written by Jagdish Mehra and Helmut Rechenberg in 1982 for a real sense of the times. They refer to Joseph “JJ” Thomson, Wilhelm Röntgen, Henri Becquerel, Marie and Pierre Curie, Ernie Rutherford, Henri Poincaré, Charles Barkla, Charles Sadler, William Henry Bragg, William Lawrence Bragg, and many others. Including Hendrik Lorentz, who they say was opposed to the light-quantum theory. They also talk about Walther Nernst and Johannes Stark, saying “besides Nernst and his school, Johannes Stark (1874-1957) had been the most active supporter of quantum theory”. Stark wrote a paper on the radiation of canal rays in hydrogen and “received encouraging support from Albert Einstein and Arnold Sommerfeld”. As for Nernst, see Wikipedia. He “read a paper of Einstein’s on the quantum mechanics of specific heats at cryogenic temperatures and was so impressed that he traveled all the way to Zurich to visit him in person”. That was in 1910. Nernst was highly regarded. His visit gave Einstein’s status a boost. The saying goes that “Einstein must be a clever fellow if the great Nernst comes all the way from Berlin to Zurich to talk to him”. It perhaps gave Einstein’s quantum confidence a boost too. In a letter to Jakob Laub, Einstein said this: “For me, the theory of quanta is a settled matter. My predictions regarding the specific heats are apparently being brilliantly confirmed. Nernst, who has just been here to see me, and Rubens are busily engaged in the experimental verification, so that we will soon know where we stand”.
The first Solvay conference
Nernst made a big difference. See the origins of the quantum theory written by Cathryn Carson in the year 2000. She says “it took the sudden attention of the physical chemist Walther Nernst to bring quantum theories of specific heats to general significance”. She also says “it was no accident, and to a large degree Nernst’s doing, that the first Solvay Congress in 1911 dealt precisely with radiation theory and quanta”. And that “if the quantum was born in 1900, the Solvay meeting was, so to speak, its social debut”. Norbert Straumann gives a partial account in his essay on the first Solvay congress in 1911. He says Ernest Solvay became very rich man from soda production, and was eager to discuss his ideas on natural science with some of Europe’s top physicists. He also says “Walther Nernst made clever use of this interest, and suggested that Solvay may fund an elite gathering at which leading scientists would listen to his ideas on gravitation, Brownian motion, radioactivity, etc”. Straumann tells us that Nernst was the organizer and Lorentz was the chairman, and that “Solvay was not present at the time the photo was taken; his photo was pasted onto this one for the official release (resulting in a rather big head)”.
 Solvay conference 1911, public domain image, see Wikipedia commons
Solvay conference 1911, public domain image, see Wikipedia commons
Straumann also says Einstein was completely isolated with his concept of a wave-particle duality for the free electromagnetic field, and that Max Planck “was for many years against Einstein’s light quanta”. Straumann also reports Einstein’s opinion of the conference. In his subsequent letter to Heinrich Zangger, Einstein said Lorentz was a marvel of intelligence and tact, and the most intelligent among the theoreticians present, whilst Poincaré was negative and Planck stuck stubbornly to preconceived opinions. In his letter to Michele Besso, Einstein said “the congress in Brussels resembled the lamentations on the ruins of Jerusalem. Nothing positive has come out of it”. That’s true enough, but henceforth other physicists did take an interest in the so-called quantum theory.
Rutherford’s model of the atom
As Mehra and Rechenberg say in their book, after the first Solvay conference in 1911 “the interest in quantum theory and the phenomena associated with it grew rapidly beyond the formerly limited circle of Planck, Einstein, Stark, and Nernst”. Contributors included Max Born, Theodore von Kármán, and Peter Debye, who improved on Einstein’s specific heat. And of course, one of the attendees at the Solvay conference was Ernest Rutherford, who had come up with a new model of the atom. His 1911 paper was on the scattering of α and β particles by matter and the structure of the atom. The crucial point was that the observations of Geiger and Marsden “indicate that some of the α particles, about 1 in 20,000 were turned through an average angle of 90 degrees in passing through a layer of gold foil about 0.00004 cm thick”. Rutherford referred to JJ Thomson’s 1904 plum-pudding model and Hantaro Nagaoka’s 1904 Saturnian model, which was partially vindicated. The atom had a positively charged centre. A nucleus. Rutherford said further consideration would be reserved for a later paper, but unfortunately he didn’t write it. Niels Bohr wrote it instead.
“Rutherford said further consideration would be reserved for a later paper, but unfortunately he didn’t write it. ” – actually, presumably fortunately, for he must have realised that the model was incorrect.
I wish he’d written it Jim. Things might be very different today if he had.