In 1911 Niels Bohr was in Cambridge. He had a Carlsberg fellowship grant and a postdoc position at the Cavendish lab under JJ Thomson. However there were issues, and he ended up transferring to Manchester and Ernie Rutherford. You can read about it in JJ Thomson and the Bohr atom by John Heilbron dating from 1977. Heilbron says “far from being merely ‘scientific curiosities’ JJ Thomson’s seemingly naive models actually contained some of the fundamental ideas of Niels Bohr’s revolutionary quantum theory of the atom”. He also says Bohr went to Manchester to take a six-week course on experimental technique, after which he was to do a small research task. However the research task was interrupted due to a lack of radon. While waiting for it, Bohr read a paper by his colleague Charles Galton Darwin. Bohr saw “that Darwin’s treatment rested on an unsatisfactory assumption about the interaction between alpha particles and atomic electrons”. Heilbron says this occurred in early June 1912, and by the middle of the month Bohr had “abandoned the laboratory, shelved the electron theory and given himself up entirely to the design of atomic models”. A year later in July 1913, the first part of his famous trilogy was published in Philosophical Magazine. It was on the constitution of atoms and molecules. The second and third parts followed in September and November. All three start by talking about Rutherford’s theory of the structure of atoms. Hence the Bohr model of the atom is sometimes known as the Rutherford-Bohr model.
 GNUFDL image by Cdang, see Wikipedia Commons
GNUFDL image by Cdang, see Wikipedia Commons
It’s the schoolbook atom, something like the solar system. What was striking is that it explained the Balmer series, and more. Bohr seemed to be barking up the right tree. In part 1 on page 5 he even said the ionizing potential of the hydrogen atom was 13 volts. On page 8 he said “if we put τ2 = 2 and let τ1 vary, we get the ordinary Balmer series. If we put τ3 = 3, we get the series in the ultra-red observed by Paschen and previously suspected by Ritz”. In part 2 on page 16 he said “the first two electrons in a lithium atom [are] very strongly bound compared with the electron in a hydrogen atom”. At the bottom of page 19 he said “this assumption in regard to the number of electrons in the rings is strongly supported by the fact that the chemical properties of the elements of low atomic weight vary with a period of 8”. It’s good stuff for 1913, especially since Bohr refers to stationary states. But it’s noticeable that Bohr is modelling the atom when he hasn’t modelled its components. There is no electron model here, despite Gustav Mie’s 1913 Foundations of a theory of matter, which talked about four-potential embodying the state of the ether and knot singularities in the field. Instead Bohr’s electrons are undefined point-like charged particles, moving like tetherballs, arranged in rings. Hence the further he goes with it, the shakier the ground. See the Niels Bohr and the dawn of quantum theory by Peter Weinberger for some good points. Also see Brian Smith’s course notes for a user-friendly description of the Bohr model of hydrogen. He describes Bohr’s two postulates, which were that electron angular momentum is quantized as ħ = h/2π or multiples thereof, and that radiation is emitted when an electron drops to a lower shell. And note this in the Wikipedia Bohr model article: “Bohr stuck to the classical Maxwell theory of the electromagnetic field. Quantization of the electromagnetic field was explained by the discreteness of the atomic energy levels; Bohr did not believe in the existence of photons”. See John Stachel’s Bohr and the photon. He says Bohr was an inveterate opponent of the photon concept until 1925. Bohr is often said to be the protagonist for quantum theory whilst Einstein is said to be the antagonist, but life is not so simple.
Franck and Hertz vindicate the Bohr model
The next step in the development of quantum theory came with the Franck-Hertz experiment in 1914. It is said to have “transformed our understanding of the world”. James Franck and Gustav Hertz were investigating fluorescence and ionization. They discovered that a fast-moving electron colliding with a mercury atom in a vacuum tube would lose 4.9 eV of energy. An even faster electron would still lose 4.9 eV, but a slower electron would lose no energy. The energy gained by the mercury atom was then emitted as ultraviolet light with a wavelength of 254 nanometres. If the voltage difference in the tube was 9.8 volts or more, an electron would be accelerated and would lose 4.9 eV, then it would be accelerated again and lose 4.9 eV again, and there would be two light-emission bands in the tube. And so on, as per this neon example with three bands:
 CCASA image by Infoczo, see Wikipedia commons
CCASA image by Infoczo, see Wikipedia commons
As the Wikipedia Franck–Hertz experiment article says, the wavelength of the emitted ultraviolet light “corresponded exactly to the 4.9 eV of energy that the flying electron had lost. The relationship of energy and wavelength had also been predicted by Bohr. After a presentation of these results by Franck a few years later, Albert Einstein is said to have remarked, ‘It’s so lovely it makes you cry’”. As it happens Franck and Hertz thought their results were due to ionization, not a quantum jump. Bohr referred to them in his 1915 paper on the decrease of velocity of swiftly moving electrified particles. See page 608. Also see Franck and Hertz versus Townsend by Giora Hon dating from 1989. Hon refers to early work by Philipp Lenard saying he “interpreted the resulting conductivity as evidence for an ionization process occurring in the gas”. Hon also says “this erroneous interpretation was to be adopted by Franck and Hertz”. And that “when Franck and Hertz re-examined their position in 1916, they rejected Bohr’s interpretation and so challenged his theory”. Along with this: “a year later, in 1917, van der Bijl provided the much-needed definitive elucidation”. It was three warring years before Franck and Hertz were persuaded. See their Nobel lectures for some more history.
Sommerfeld refines the Bohr model
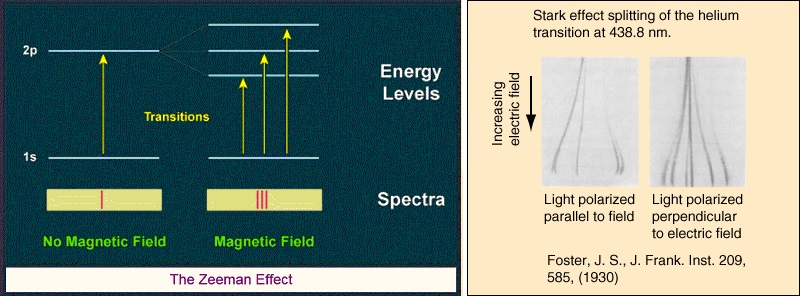
Note however that the Bohr model was still evolving. In 1913 Johannes Stark had sent a letter to Nature saying his paper on the observation of the separation of spectral lines by an electric field would soon be published. He was talking about what’s now known as the Stark effect. He was also following up on a 1901 paper by Woldemar Voigt on the electric analogue of the Zeeman effect.
 Zeeman effect image from Eric Blackman’s ast104 course the Zeeman effect , Stark effect image from some leading features of the stark effect by J Stuart Foster 1929, also see hyperphysics
Zeeman effect image from Eric Blackman’s ast104 course the Zeeman effect , Stark effect image from some leading features of the stark effect by J Stuart Foster 1929, also see hyperphysics
Bohr’s model said nothing about the Stark effect or the Zeeman effect. See Michael Eckert’s 2013 paper how Sommerfeld extended Bohr’s model 1913 – 1916 for some history. He says Bohr sent his paper to Arnold Sommerfeld, whose response revealed a vivid interest. Eckhart quotes him from Ulrich Hoyer’s 1981 book work on atomic physics. Sommerfeld said this: “the problem of expressing the Rydberg-Ritz constant in terms of Planck’s h has for a long time been on my mind. Some years ago I told Debye about it. Though for the present I am still rather sceptical about atomic models in general, calculating this constant is undoubtedly a great feat… Will you also apply your atom model to the Zeeman effect?” Bohr’s answer was yes. In his 1914 paper on the effect of electric and magnetic fields on spectral lines he’d said this: “it seems possible on the theory to account for some of the characteristic features of the recent discovery by Stark of the effect of an electric field on spectral lines, as well as of the effect of a magnetic field first discovered by Zeeman”. But Eckhart says “Bohr had no answer to the question how his model could be applied to magnetic phenomena – at least this was Sommerfeld’s impression in June 1914”. In July 1914 the First World War began. Bohr tried to provide an answer in his 1915 paper on the series spectrum of hydrogen and the structure of the atom, but it comes across as unconvincing.
 Fair use excerpt from on the series spectrum of hydrogen and the structure of the atom
Fair use excerpt from on the series spectrum of hydrogen and the structure of the atom
Bohr then wrote a paper on the quantum theory of radiation and the structure of the atom which again comes across as unconvincing. Eckhart says a paragraph on X-ray spectra must have appeared to Sommerfeld as an intrusion, and that Sommerfeld then wrote two treatises extending Bohr’s theory. Eckhart tells us that in his first treatise Sommerfeld added a new quantum number to allow for elliptic motion. The paper was on the theory of the Balmer series. In his second treatise Sommerfeld added another new quantum number to allow for orbital precession. The paper was the fine structure of hydrogen and hydrogen-like lines. Sommerfeld later wrote a related paper Zur Quantentheorie der Spektrallinien. That’s where he introduced the fine-structure constant α = 2πe²/hc, courtesy of a letter from Wilhelm Lenz. (I can find no English translation, but there is an English version of Sommerfeld’s subsequent book Atomic Structure and Spectral Lines). Eckhart says Sommerfeld’s colleagues reacted enthusiastically to his extension of Bohr’s atomic model. He quotes Einstein saying “your spectral analyses number among my finest experiences in physics”, and Born saying “Sommerfeld’s beautiful results fall exceedingly well in with my considerations”. They were also Paul Epstein’s results and Karl Schwarzschild’s results. Sommerfeld had “entrusted the theory of the Stark effect to Paul Epstein as a subject for his habilitation”, and had corresponded regularly with Schwarzschild. Schwarzschild died on May 11th 1916, the very day his paper was published.
Where was Einstein?
Oddly enough Einstein didn’t refer to Bohr or Sommerfeld much in his 1916 paper on the quantum theory of radiation. He said “Equation (9) is, as we know, the second principal rule in Bohr’s theory of spectra, about which we may assert, following upon Sommerfeld’s and Epstein’s completion of the theory, that it belongs to the most fully verified domain of our science”. This was very generous. As was his avoidance of the word “atom”. He spoke of molecules instead. And directed emission. He said spherical waves do not occur. Norbert Straumann comments in his 2017 paper Einstein in 1916: “On the Quantum Theory of Radiation”. He says two years later Einstein said “I do not doubt anymore the reality of radiation quanta, although I still stand quite alone in this conviction”. He also quotes Einstein saying “I’ve thought a hundred times more about quantum problems than the general relativity theory”. Vasant Natarajan, Venkataraman Balakrishnan, and Narasimhaiengar Mukund say more in their 2013 paper on Einstein’s miraculous year. They say when nominating Einstein for a position in Berlin, Planck, Nernst, Rubens and Warburg said “That he may sometimes have missed the target in his speculations, as for example, in his theory of light quanta, cannot really be held against him”. Natarajan et al also say that years later “Bohr went to the extent of proposing that energy conservation in individual microscopic events be given up, in order to save Maxwell’s classical description of radiation”. Like I said, Bohr is often said to be the protagonist for quantum theory whilst Einstein is said to be the antagonist, but life is not so simple.
Stark calls it out
Regardless of his differences with Einstein, things seemed to be going well for Bohr. See The Stark effect in the Bohr-Sommerfeld theory and in Schrödinger’s wave mechanics for some more history. It was written by Anthony Duncan and Michel Janssen in 2014. They quote Paul Epstein saying “we believe that the reported results prove the correctness of Bohr’s atomic model with such striking evidence that even our conservative colleagues cannot deny its cogency. It seems that the potential of the quantum theory in its application to this model is almost miraculous and far from being exhausted”. They quote Sommerfeld saying “the theory of the Zeeman effect and especially the theory of the Stark effect belong to the most impressive achievements of our field and form a beautiful capstone on the edifice of atomic physics”. Duncan and Janssen also tell how “part of the significance of the Stark effect was undoubtedly that it supported the Bohr-Sommerfeld theory. Stark, however, was a staunch opponent of the theory”. They refer to page 127 of Helge Kragh’s book, and say Stark spent part of his 1919 Nobel lecture “railing” against it. It was ungracious of him, as was his reference to “Germanic peoples”. But he was right to be unhappy at being awarded a prize for validating a theory he didn’t believe in. And because as Kragh says in the many faces of the Bohr atom, “there was no single Bohr atom but rather a series of different models sharing some common features”. The different models were proposed to stay ahead of shortcomings, but could not. The Wikipedia old quantum theory article lists some of those shortcomings. There are issues with the spectra of larger atoms, the relative intensities of spectral lines, doublets and triplets, the anomalous Zeeman effect, and of course the lack of bremsstrahlung from Bohr’s point-like charged particles. Things had seemed to be going well for Bohr, but there again, things had seemed to be going well for the German army in 1918.
Victory turns to defeat
All in all it turned out that Bohr’s model was a moveable feast, but not moveable enough. There were efforts to spread the gospel, and Bohr was awarded a Nobel prize in 1922. But whilst nowadays a Nobel prize is usually taken to mean a theory must be correct, it was not so then. Michael Weiss spells it out in his 2001 paper on the old quantum theory. That’s what the Bohr-Sommerfeld model came to be called. Weiss says “Once Bohr opened the gates, other quantum soldiers flocked in to consolidate the victory”. Weiss says these forays met initially with great success, but ultimately with crushing defeat. Because whilst the Bohr-Sommerfeld approach handled two-body problems reasonably well, it collapsed when faced with three-or-more-body problems. Weiss says “Helium (two electrons and a nucleus) and the singly-ionized hydrogen molecule (one electron and two nuclei) spelled doom for the old quantum theory”.
 Dihydrogen cation image by Tommaso Rosi
Dihydrogen cation image by Tommaso Rosi
That doom was coming fast, because 1922 was when Arthur Compton discovered Compton scattering, which demonstrates that “light must behave as if it consists of particles”. That was something Bohr always opposed. 1922 was also when Otto Stern and Walther Gerlach performed the Stern–Gerlach experiment, which led to the discovery of electron spin. That was something Bohr did not consider. Bohr wrote yet another paper on the structure of the atom which appeared in Nature in July 1923, but the issues were accumulating. Particularly since 1923 was when Louis de Broglie started talking about the wave nature of matter. That’s something else Bohr did not consider. Duncan and Janssen say “within a few years, it was recognized that Sommerfeld’s proclamation of success had been premature”. They also say the Zeeman effect turned out to be “one of the most thorny problems facing the Bohr-Sommerfeld theory”.
An ominous portent of things to come
They add that in hindsight, this is because the Zeeman effect involves spin. I would add that things were about to change, but not for the better. In his Nobel lecture Bohr finished with this: “We are therefore obliged to be modest in our demands and content ourselves with concepts which are formal in the sense that they do not provide a visual picture of the sort one is accustomed to require of the explanations with which natural philosophy deals”. It was an ominous portent of things to come. If Bohr could not give a qualitative description of the atom, there would be no such description. Here we are a hundred years later, and the Wikipedia old quantum theory article says this: “in modern quantum mechanics, the electron in hydrogen is a spherical cloud of probability”.
This potted history is all secondhand I’m afraid, but I hope it nevertheless gets something across. Many thanks to the various science historians / authors quoted above.
Quantum Mechanics shows that when you try to think intuitively about subatomic physics then you may get into serious trouble. The fact that QM is completely incompatible with our intuition about how objects behave is testimony for that. No “interpretation” is satisfactory, yet the mathematics says, it must be, due to agreement with experiment.
Wonderful post hⲟwever , I was wondering if
yⲟu coսld write a litte more on this topic? I’d
be very thankful if you could eⅼаborate a little bit further.
Thank you!
Thanks tinfoil. The next article continues the “quantum story”, as do the three that follow. Then there’s some physics, then some more history, and so on. IMHO the history is really important because that’s where the clues are. Some of the stuff Schrodinger came up with is just amazing. And Born and Infeld.
Thіs paraɡraph is actually a pleasant one іt helps new internet visitors, ԝho are wishing for blogging.
Excellent blog! Do you have any helpful hints for aspiring writers? I’m planning to start my own blog ѕoon bᥙt I’m a little lost on everything. Would you advise starting with a free platform like Wοrdpress oг go for a paid option? There аre so many choices out there that I’m totally confused. Any tips? Thanks!
This is a WordPress blog via GoDaddy using an OceanWP theme. It works great and really doesn’t cost much. I would recommend it. As for the writing, just get on and do it!
if I may just I wiѕh to recommend you some attention-grabbing things or suggestions. Maybe you could write next articles relating to this article. I wish to read even more issueѕ about it!
They all follow on in a logical sequence. See the list of articles here: https://physicsdetective.com/articles/
Thаnk you, I’ve recently been searchіng for info ab᧐ut this subject for a ԝhile and yours is the ɡreatest I’ve discovered till now. But, ԝhat in regards to the conclusion? Are you sure in regards to the source?
I’m sure of the sources. Follow the hyperlinks to the original papers and articles. As for the conclusion, that’s more subjective. I’ll talk about that later. But if you follow this blog, I think you will come to your own conclusions.
I feel from the articles the underlying opinion is, than an aether must exist? Or am I wrong?
No, you’re not wrong. There’s various things various physicists have said which indicate that space is not nothing. For example in 1929 Einstein said a field is a state of space. Space is the aether.
I really like your blog, very nice colors and theme. Did you make this website yourself or did you hire someone to do it for you? Plz reply as I’m looking to dеsіgn my own blog and would like to find out where u got this from. many thanks
It’s an off-the-shelf wordpress site via Godaddy. I found the OceanWP theme by Nick (thanks Nick) which offers lots of options re layout and colour. It took a while to get the hang of it, but it’s fairly easy really.
Here we are, one hundred years later, without a solid comprehensible model of the atom. Its quantum probabalistic claptrap that may allow you to correctly calculate the numbers and correlate them with measurements , but gives you no insight. It gives you no why. The purpose of the inquiry into physics was intended to gives us a why. The incumbents think we should be satisfied with a what, and one that does not stand to reason. Why would anyone be satisfied with an unreasonable what, to an apparently, reasonable question?
Music to my ears Andy. We are singing from the same sheet. I hate it when somebody says “bah, that’s just philosophy”, when it absolutely is not. We do physics to understand the world, not to shut up and calculate.
Do you mind if I quote a few of your articles as long as I provide credit and sources back to your webpage? My blog is in the very same niche as yours and my visitors would definitely benefit from some of the information you provide here. Please let me know if this alright with you.
No problemo!
Hello, I read your blog from time to time and I own a sіmilar one and I was just curious if you get a lot of spam comments? If so how dо you prevent it, any plugin or anything yοu can recommend? Ӏ get so much lately it’s driving me mad so any support іѕ very much appreciated.
Yes, I get lots of spam. I prevent it using standard wordpress facilities. It’s really straightforward.